Counter Electrode Reactions-Important Stumbling Blocks on the Way to a Working Electro-organic Synthesis
- PMID: 35668714
- PMCID: PMC9828107
- DOI: 10.1002/anie.202204140
Counter Electrode Reactions-Important Stumbling Blocks on the Way to a Working Electro-organic Synthesis
Abstract
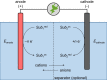
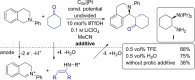
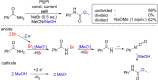
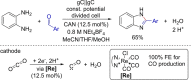
Over the past two decades, electro-organic synthesis has gained significant interest, both in technical and academic research as well as in terms of applications. The omission of stoichiometric oxidizers or reducing agents enables a more sustainable route for redox reactions in organic chemistry. Even if it is well-known that every electrochemical oxidation is only viable with an associated reduction reaction and vice versa, the relevance of the counter reaction is often less addressed. In this Review, the importance of the corresponding counter reaction in electro-organic synthesis is highlighted and how it can affect the performance and selectivity of the electrolytic conversion. A selection of common strategies and unique concepts to tackle this issue are surveyed to provide a guide to select appropriate counter reactions for electro-organic synthesis.
Keywords: Electrochemistry; Hydrogen; Oxygen; Paired Electrolyses; Sacrificial Anodes.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















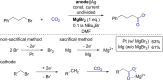

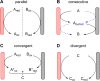
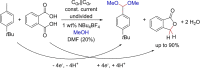


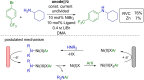
References
-
- None
-
- Waldvogel S. R., Janza B., Angew. Chem. Int. Ed. 2014, 53, 7122–7123; - PubMed
- Angew. Chem. 2014, 126, 7248–7249;
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

