Interleukin 15 modulates the effects of poly I:C maternal immune activation on offspring behaviour
- PMID: 35668725
- PMCID: PMC9166394
- DOI: 10.1016/j.bbih.2022.100473
Interleukin 15 modulates the effects of poly I:C maternal immune activation on offspring behaviour
Abstract
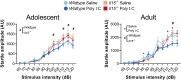
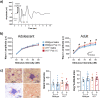
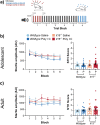
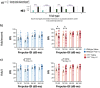
Maternal infections during pregnancy are linked with an increased risk for disorders like Autism Spectrum Disorder and schizophrenia in the offspring. Although precise mechanisms are still unclear, clinical and preclinical evidence suggest a strong role for maternal immune activation (MIA) in the neurodevelopmental disruptions caused by maternal infection. Previously, studies using the Polyinosinic:Polycytidylic (Poly I:C) MIA preclinical model showed that cytokines like Interleukin 6 (Il6) are important mediators of MIA's effects. In this study, we hypothesized that Il15 may similarly act as a mediator of Poly I:C MIA, given its role in the antiviral immune response. To test this hypothesis, we induced Poly I:C MIA at gestational day 9.5 in wildtype (WT) and Il15 -/- rat dams and tested their offspring in adolescence and adulthood. Poly I:C MIA and Il15 knockout produced both independent and synergistic effects on offspring behaviour. Poly I:C MIA decreased startle reactivity in adult WT offspring but resulted in increased adolescent anxiety and decreased adult locomotor activity in Il15 -/- offspring. In addition, Poly I:C MIA led to genotype-independent effects on locomotor activity and prepulse inhibition. Finally, we showed that Il15 -/- offspring exhibit distinct phenotypes that were unrelated to Poly I:C MIA including altered startle reactivity, locomotion and signal transduction in the auditory brainstem. Overall, our findings indicate that the lack of Il15 can leave offspring either more or less susceptible to Poly I:C MIA, depending on the phenotype in question. Future studies should examine the contribution of fetal versus maternal Il15 in MIA to determine the precise developmental mechanisms underlying these changes.
Keywords: Age; Anxiety; Autism spectrum disorder; Interleukin 15; Maternal immune activation; Poly I:C; Pregnancy; Schizophrenia; Social behaviour; Startle.
© 2022 The Authors.
Figures


Similar articles
-
The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model.Brain Behav Immun. 2014 Nov;42:138-46. doi: 10.1016/j.bbi.2014.06.013. Epub 2014 Jun 26. Brain Behav Immun. 2014. PMID: 24973728
-
Investigating behavioral phenotypes related to autism spectrum disorder in a gene-environment interaction model of Cntnap2 deficiency and Poly I:C maternal immune activation.Front Neurosci. 2023 Mar 14;17:1160243. doi: 10.3389/fnins.2023.1160243. eCollection 2023. Front Neurosci. 2023. PMID: 36998729 Free PMC article.
-
Role of Polyinosinic:Polycytidylic Acid-Induced Maternal Immune Activation and Subsequent Immune Challenge in the Behaviour and Microglial Cell Trajectory in Adult Offspring: A Study of the Neurodevelopmental Model of Schizophrenia.Int J Mol Sci. 2021 Feb 4;22(4):1558. doi: 10.3390/ijms22041558. Int J Mol Sci. 2021. PMID: 33557113 Free PMC article.
-
Maternal Immune Activation by Poly I:C as a preclinical Model for Neurodevelopmental Disorders: A focus on Autism and Schizophrenia.Neurosci Biobehav Rev. 2020 Jun;113:546-567. doi: 10.1016/j.neubiorev.2020.04.012. Epub 2020 Apr 19. Neurosci Biobehav Rev. 2020. PMID: 32320814 Review.
-
Virus-Induced Maternal Immune Activation as an Environmental Factor in the Etiology of Autism and Schizophrenia.Front Neurosci. 2022 Apr 12;16:834058. doi: 10.3389/fnins.2022.834058. eCollection 2022. Front Neurosci. 2022. PMID: 35495047 Free PMC article. Review.
Cited by
-
Characterizing maternal isolation-induced ultrasonic vocalizations in a gene-environment interaction rat model for autism.Genes Brain Behav. 2023 Jun;22(3):e12841. doi: 10.1111/gbb.12841. Epub 2023 Feb 7. Genes Brain Behav. 2023. PMID: 36751016 Free PMC article.
-
Prenatal allergic inflammation in rats confers sex-specific alterations to oxytocin and vasopressin innervation in social brain regions.Horm Behav. 2024 Jan;157:105427. doi: 10.1016/j.yhbeh.2023.105427. Epub 2023 Sep 22. Horm Behav. 2024. PMID: 37743114 Free PMC article.
References
-
- Abdallah M.W., Larsen N., Grove J., Nørgaard-Pedersen B., Thorsen P., Mortensen E.L., Hougaard D.M. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World J. Biol. Psychiatr. 2013;14:528–538. doi: 10.3109/15622975.2011.639803. - DOI - PubMed
-
- Alleva D.G., Kaser S.B., Monroy M.A., Fenton M.J., Beller D.I. IL-15 functions as a potent autocrine regulator of macrophage proinflammatory cytokine production: evidence for differential receptor subunit utilization associated with stimulation or inhibition. J. Immunol. 1997;159:2941–2951. - PubMed
-
- Atladóttir H.Ó., Henriksen T.B., Schendel D.E., Parner E.T. Using maternally reported data to investigate the association between early childhood infection and autism spectrum disorder: the importance of data source. Paediatr. Perinat. Epidemiol. 2012;26:373–385. doi: 10.1111/j.1365-3016.2012.01296.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources

