Vitrification and Rewarming of Magnetic Nanoparticle-Loaded Rat Hearts
- PMID: 35668819
- PMCID: PMC9164386
- DOI: 10.1002/admt.202100873
Vitrification and Rewarming of Magnetic Nanoparticle-Loaded Rat Hearts
Abstract
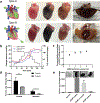
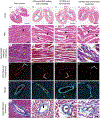
To extend the preservation of donor hearts beyond the current 4-6 h, this paper explores heart cryopreservation by vitrification-cryogenic storage in a glass-like state. While organ vitrification is made possible by using cryoprotective agents (CPA) that inhibit ice during cooling, failure occurs during convective rewarming due to slow and non-uniform rewarming which causes ice crystallization and/or cracking. Here an alternative, "nanowarming", which uses silica-coated iron oxide nanoparticles (sIONPs) perfusion loaded through the vasculature is explored, that allows a radiofrequency coil to rewarm the organ quickly and uniformly to avoid convective failures. Nanowarming has been applied to cells and tissues, and a proof of principle study suggests it is possible in the heart, but proper physical and biological characterization especially in organs is still lacking. Here, using a rat heart model, controlled machine perfusion loading and unloading of CPA and sIONPs, cooling to a vitrified state, and fast and uniform nanowarming without crystallization or cracking is demonstrated. Further, nanowarmed hearts maintain histologic appearance and endothelial integrity superior to convective rewarming and indistinguishable from CPA load/unload control hearts while showing some promising organ-level (electrical) functional activity. This work demonstrates physically successful heart vitrification and nanowarming and that biological outcomes can be expected to improve by reducing or eliminating CPA toxicity during loading and unloading.
Keywords: cryopreservation; heart; iron oxide nanoparticle; radio frequency warming; vitrification.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures





Similar articles
-
A guide to successful mL to L scale vitrification and rewarming.Cryo Letters. 2022 Nov-Dec;43(6):316-321. Cryo Letters. 2022. PMID: 36629824 Free PMC article. Review.
-
Physical vitrification and nanowarming at human organ scale to enable cryopreservation.bioRxiv [Preprint]. 2024 Nov 11:2024.11.08.622572. doi: 10.1101/2024.11.08.622572. bioRxiv. 2024. PMID: 39605575 Free PMC article. Preprint.
-
Vitrification and Nanowarming of Kidneys.Adv Sci (Weinh). 2021 Oct;8(19):e2101691. doi: 10.1002/advs.202101691. Epub 2021 Aug 11. Adv Sci (Weinh). 2021. PMID: 34382371 Free PMC article.
-
Cryopreservation of Whole Rat Livers by Vitrification and Nanowarming.Ann Biomed Eng. 2023 Mar;51(3):566-577. doi: 10.1007/s10439-022-03064-2. Epub 2022 Oct 1. Ann Biomed Eng. 2023. PMID: 36183025 Free PMC article.
-
Review of Rewarming Methods for Cryopreservation.Biopreserv Biobank. 2024 Aug;22(4):304-311. doi: 10.1089/bio.2023.0015. Epub 2023 Sep 26. Biopreserv Biobank. 2024. PMID: 37751240 Review.
Cited by
-
A guide to successful mL to L scale vitrification and rewarming.Cryo Letters. 2022 Nov-Dec;43(6):316-321. Cryo Letters. 2022. PMID: 36629824 Free PMC article. Review.
-
Cryopreservation of tissues and organs: present, bottlenecks, and future.Front Vet Sci. 2023 May 25;10:1201794. doi: 10.3389/fvets.2023.1201794. eCollection 2023. Front Vet Sci. 2023. PMID: 37303729 Free PMC article. Review.
-
Physical vitrification and nanowarming at human organ scale to enable cryopreservation.bioRxiv [Preprint]. 2024 Nov 11:2024.11.08.622572. doi: 10.1101/2024.11.08.622572. bioRxiv. 2024. PMID: 39605575 Free PMC article. Preprint.
-
Thermal Analyses of Nanowarming-Assisted Recovery of the Heart From Cryopreservation by Vitrification.J Heat Transfer. 2022 Mar 1;144(3):031202. doi: 10.1115/1.4053105. Epub 2022 Jan 18. J Heat Transfer. 2022. PMID: 35833152 Free PMC article.
-
Thermomechanical stress analyses of nanowarming-assisted recovery from cryopreservation by vitrification in human heart and rat heart models.PLoS One. 2023 Aug 16;18(8):e0290063. doi: 10.1371/journal.pone.0290063. eCollection 2023. PLoS One. 2023. PMID: 37585446 Free PMC article.
References
-
- United Network for Organ Sharing (UNOS) data, https://unos.org/data/ (accessed: December 2019).
-
- Jahania MS, Sanchez JA, Narayan P, Lasley RD, Mentzer RM Jr., Ann. Thorac. Surg. 1999, 68, 1983. - PubMed
-
- Ely D, Dunphy G, Dollwet H, Richter H, Sellke F, Azodi M, Free Radicals Biol. Med. 1992, 12, 479. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
