Human Cardiac Progenitor Cell-Derived Extracellular Vesicles Exhibit Promising Potential for Supporting Cardiac Repair in Vitro
- PMID: 35669580
- PMCID: PMC9163838
- DOI: 10.3389/fphys.2022.879046
Human Cardiac Progenitor Cell-Derived Extracellular Vesicles Exhibit Promising Potential for Supporting Cardiac Repair in Vitro
Abstract
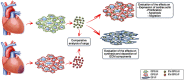
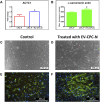
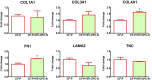
Although human Cardiac Progenitor Cells (hCPCs) are not retained by host myocardium they still improve cardiac function when injected into ischemic heart. Emerging evidence supports the hypothesis that hCPC beneficial effects are induced by paracrine action on resident cells. Extracellular vesicles (EVs) are an intriguing mechanism of cell communication based on the transport and transfer of peptides, lipids, and nucleic acids that have the potential to modulate signaling pathways, cell growth, migration, and proliferation of recipient cells. We hypothesize that EVs are involved in the paracrine effects elicited by hCPCs and held accountable for the response of the infarcted myocardium to hCPC-based cell therapy. To test this theory, we collected EVs released by hCPCs isolated from healthy myocardium and evaluated the effects they elicited when administered to resident hCPC and cardiac fibroblasts (CFs) isolated from patients with post-ischemic end-stage heart failure. Evidence emerging from our study indicated that hCPC-derived EVs impacted upon proliferation and survival of hCPCs residing in the ischemic heart and regulated the synthesis and deposition of extracellular-matrix by CFs. These findings suggest that beneficial effects exerted by hCPC injection are, at least to some extent, ascribable to the delivery of signals conveyed by EVs.
Keywords: cardiac regenerative medicine; extracellular vesicles; human cardiac fibroblasts; human cardiac progenitor cells; paracrine effects.
Copyright © 2022 Romano, Belviso, Sacco, Cozzolino, Nurzynska, Amarelli, Maiello, Sirico, Di Meglio and Castaldo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Barile L., Lionetti V., Cervio E., Matteucci M., Gherghiceanu M., Popescu L. M., et al. (2014). Extracellular Vesicles from Human Cardiac Progenitor Cells Inhibit Cardiomyocyte Apoptosis and Improve Cardiac Function after Myocardial Infarction. Cardiovasc. Res. 103, 530–541. 10.1093/cvr/cvu167 - DOI - PubMed
LinkOut - more resources
Full Text Sources

