Role of COVID-19 Vaccines in SARS-CoV-2 Variants
- PMID: 35669787
- PMCID: PMC9165056
- DOI: 10.3389/fimmu.2022.898192
Role of COVID-19 Vaccines in SARS-CoV-2 Variants
Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is a threat to the health of the global population. As the result of a global effort in the determination of origin, structure, and pathogenesis of SARS-CoV-2 and its variants, particularly such the variant of concern as Delta Variant and Omicron Variant, the understanding of SARS-CoV-2 are deepening and the development of vaccines against SARS-CoV-2 are ongoing. Currently, AstraZeneca-Vaxzevria/SII-Covishield vaccine, Janssen-Ad26.COV2.S vaccine, Moderna-mRNA-1273 vaccine, Pfizer BioNTech-Comirnaty vaccine and Sinovac-CoronaVac vaccine have been listed as WHO Emergency Use Listing (EUL) Qualified Vaccines by WHO. Because of the antigen escape caused by the mutation in variants, the effectiveness of vaccines, which are currently the main means of prevention and treatment, has been affected by varying degrees. Herein, we review the current status of mutations of SARS-CoV-2 variants, the different approaches used in the development of COVID-19 vaccines, and COVID-19 vaccine effectiveness against SARS-CoV-2 variants.
Keywords: COVID-19; COVID-19 vaccines; Delta variant; Omicron variant; SARS-CoV-2.
Copyright © 2022 Zhou, Zhu and Chu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
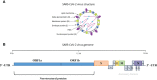
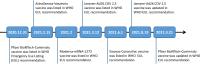
Similar articles
-
Effectiveness of CoronaVac, ChAdOx1 nCoV-19, BNT162b2, and Ad26.COV2.S among individuals with previous SARS-CoV-2 infection in Brazil: a test-negative, case-control study.Lancet Infect Dis. 2022 Jun;22(6):791-801. doi: 10.1016/S1473-3099(22)00140-2. Epub 2022 Apr 1. Lancet Infect Dis. 2022. PMID: 35366959 Free PMC article.
-
Comparative Effectiveness of Coronavirus Disease 2019 (COVID-19) Vaccines Against the Delta Variant.Clin Infect Dis. 2022 Aug 24;75(1):e623-e629. doi: 10.1093/cid/ciac106. Clin Infect Dis. 2022. PMID: 35137006 Free PMC article.
-
Neutralizing antibodies to SARS-CoV-2 variants of concern including Delta and Omicron in subjects receiving mRNA-1273, BNT162b2, and Ad26.COV2.S vaccines.J Med Virol. 2022 Dec;94(12):5678-5690. doi: 10.1002/jmv.28032. Epub 2022 Aug 6. J Med Virol. 2022. PMID: 35902378 Free PMC article.
-
Role of available COVID-19 vaccines in reducing deaths and perspective for next generation vaccines and therapies to counter emerging viral variants: an update.Minerva Med. 2023 Oct;114(5):683-697. doi: 10.23736/S0026-4806.23.08509-9. Epub 2023 Jun 9. Minerva Med. 2023. PMID: 37293890 Review.
-
Implication of in silico studies in the search for novel inhibitors against SARS-CoV-2.Arch Pharm (Weinheim). 2022 May;355(5):e2100360. doi: 10.1002/ardp.202100360. Epub 2022 Mar 4. Arch Pharm (Weinheim). 2022. PMID: 35244237 Free PMC article. Review.
Cited by
-
How far are the new wave of mRNA drugs from us? mRNA product current perspective and future development.Front Immunol. 2022 Sep 12;13:974433. doi: 10.3389/fimmu.2022.974433. eCollection 2022. Front Immunol. 2022. PMID: 36172353 Free PMC article. Review.
-
The End or a New Era of Development of SARS-CoV-2 Virus: Genetic Variants Responsible for Severe COVID-19 and Clinical Efficacy of the Most Commonly Used Vaccines in Clinical Practice.Vaccines (Basel). 2023 Jun 30;11(7):1181. doi: 10.3390/vaccines11071181. Vaccines (Basel). 2023. PMID: 37514997 Free PMC article. Review.
-
Acceptability for the influenza virus vector COVID-19 vaccine for intranasal spray: A cross-sectional survey in Beijing, China.Hum Vaccin Immunother. 2023 Aug 1;19(2):2235963. doi: 10.1080/21645515.2023.2235963. Hum Vaccin Immunother. 2023. PMID: 37450312 Free PMC article.
-
Hospital Outcomes in Patients Hospitalized for COVID-19 Pneumonia: The Effect of SARS-CoV-2 Vaccination and Vitamin D Status.Nutrients. 2023 Jun 30;15(13):2976. doi: 10.3390/nu15132976. Nutrients. 2023. PMID: 37447302 Free PMC article.
-
Effectiveness of pulmonary rehabilitation programmes and/or respiratory muscle training in patients with post-COVID conditions: a systematic review.Respir Res. 2024 Jun 19;25(1):248. doi: 10.1186/s12931-024-02857-4. Respir Res. 2024. PMID: 38890699 Free PMC article.
References
-
- World Health Organization (WHO) . WHO Coronavirus (COVID-19) Dashboard (2021). Available at: https://covid19.who.int/.
-
- Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In: StatPearls. Treasure Island (FL: StatPearls Publishing; (2022). - PubMed
-
- World Health Organization (WHO) . COVID-19 Vaccine Tracker and Landscape (2021). Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand....
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

