Impact of e-cigarette aerosol on primary human alveolar epithelial type 2 cells
- PMID: 35670478
- PMCID: PMC9559034
- DOI: 10.1152/ajplung.00503.2021
Impact of e-cigarette aerosol on primary human alveolar epithelial type 2 cells
Abstract
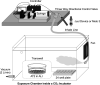
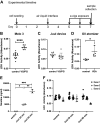
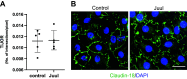
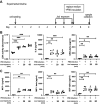
Electronic cigarettes (e-cigarettes) are designed to simulate combustible cigarette smoking and to aid in smoking cessation. Although the number of e-cigarette users has been increasing, the potential health impacts and biological effects of e-cigarettes are still not fully understood. Previous research has focused on the biological effects of e-cigarettes on lung cancer cell lines and distal airway epithelial cells; however, there have been few published studies on the effect of e-cigarettes on primary lung alveolar epithelial cells. The primary purpose of this study was to investigate the direct effect of e-cigarette aerosol on primary human lung alveolar epithelial type 2 (AT2) cells, both alone and in the presence of viral infection. The Melo-3 atomizer caused direct AT2 cell toxicity, whereas the more popular Juul pod's aerosol did not have a detectable cytotoxic effect on AT2 cells. Juul nicotine aerosol also did not increase short-term susceptibility to viral infection. However, 3 days of exposure upregulated genes central to the generation of reactive oxygen species, lipid peroxidation, and carcinogen metabolism and downregulated key innate immune system genes related to cytokine and chemokine signaling. These findings have implications for the potentially injurious impact of long-term use of popular low-power e-cigarette pods on the human alveolar epithelium. Gene expression data might be an important endpoint for evaluating the potential harmful effects of vaping devices that do not cause overt toxicity.
Keywords: EVALI; alveolar type II cells; e-cigarettes; inflammation; pulmonary edema.
Conflict of interest statement
C.S.C. has received grants and personal fees from Bayer and GlaxoSmithKline, personal fees from Boehringer Ingelheim, CSL Behring, Prometic, and Roche/Genentech. M.A.M. has received grants from Bayer Pharmaceuticals and GlaxoSmithKline, personal fees from Boehringer Ingelheim, Cerus Therapeutics, CSL Berhing, Quark Pharmaceuticals, Roche-Genentec, and Thesan Pharmaceuticals. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures

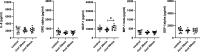


References
-
- Hammond D, Reid JL, Rynard VL, Fong GT, Cummings KM, McNeill A, Hitchman S, Thrasher JF, Goniewicz ML, Bansal-Travers M, O'Connor R, Levy D, Borland R, White CM. Prevalence of vaping and smoking among adolescents in Canada, England, and the United States: repeat national cross sectional surveys. BMJ 365: l2219, 2019. [Erratum in BMJ 370: m2579, 2020]. doi: 10.1136/bmj.l2219. - DOI - PMC - PubMed
-
- Lozier MJ, Wallace B, Anderson K, Ellington S, Jones CM, Rose D, Baldwin G, King BA, Briss P, Mikosz CA; Lung Injury Response Epidemiology/Surveillance Task Force. Update: demographic, product, and substance-use characteristics of hospitalized patients in a nationwide outbreak of E-cigarette, or vaping, product use-associated lung injuries—United States, December 2019. MMWR Morb Mortal Wkly Rep 68: 1142–1148, 2019. [Erratum in MMWR Morb Mortal Wkly Rep 69: 117, 2020]. doi: 10.15585/mmwr.mm6849e1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

