Evolution of genetic mechanisms regulating cortical neurogenesis
- PMID: 35670518
- PMCID: PMC9543202
- DOI: 10.1002/dneu.22891
Evolution of genetic mechanisms regulating cortical neurogenesis
Abstract
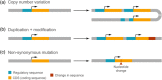
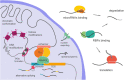
The size of the cerebral cortex increases dramatically across amniotes, from reptiles to great apes. This is primarily due to different numbers of neurons and glial cells produced during embryonic development. The evolutionary expansion of cortical neurogenesis was linked to changes in neural stem and progenitor cells, which acquired increased capacity of self-amplification and neuron production. Evolution works via changes in the genome, and recent studies have identified a small number of new genes that emerged in the recent human and primate lineages, promoting cortical progenitor proliferation and increased neurogenesis. However, most of the mammalian genome corresponds to noncoding DNA that contains gene-regulatory elements, and recent evidence precisely points at changes in expression levels of conserved genes as key in the evolution of cortical neurogenesis. Here, we provide an overview of basic cellular mechanisms involved in cortical neurogenesis across amniotes, and discuss recent progress on genetic mechanisms that may have changed during evolution, including gene expression regulation, leading to the expansion of the cerebral cortex.
Keywords: OSVZ; cerebral cortex; enhancer; ferret; intermediate progenitor; radial glia.
© 2022 The Authors. Developmental Neurobiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Amadei, G. , Zander, M. A. , Yang, G. , Dumelie, J. G. , Vessey, J. P. , Lipshitz, H. D. , Smibert, C. A. , Kaplan, D. R. , & Miller, F. D. (2015). A smaug2‐based translational repression complex determines the balance between precursor maintenance versus differentiation during mammalian neurogenesis. Journal of Neuroscience, 35, 15666–15681. 10.1523/JNEUROSCI.2172-15.2015 - DOI - PMC - PubMed
-
- Antonacci, F. , Dennis, M. Y. , Huddleston, J. , Sudmant, P. H. , Steinberg, K. M. , Rosenfeld, J. A. , Miroballo, M. , Graves, T. A. , Vives, L. , Malig, M. , Denman, L. , Raja, A. , Stuart, A. , Tang, J. , Munson, B. , Shaffer, L. G. , Amemiya, C. T. , Wilson, R. K. , & Eichler, E. E. (2014). Palindromic GOLGA8 core duplicons promote chromosome 15q13.3 microdeletion and evolutionary instability. Nature Genetics, 46, 1293–1302. 10.1038/ng.3120 - DOI - PMC - PubMed
-
- Arcila, M. L. , Betizeau, M. , Cambronne, X. A. , Guzman, E. , Doerflinger, N. , Bouhallier, F. , Zhou, H. , Wu, B. , Rani, N. , Bassett, D. S. , Borello, U. , Huissoud, C. , Goodman, R. H. , Dehay, C. , & Kosik, K. S. (2014). Novel primate miRNAs coevolved with ancient target genes in germinal zone‐specific expression patterns. Neuron, 81, 1255–1262. 10.1016/j.neuron.2014.01.017 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

