Construction of a Rhodobacter sphaeroides Strain That Efficiently Produces Hydrogen Gas from Acetate without Poly(β-Hydroxybutyrate) Accumulation: Insight into the Role of PhaR in Acetate Metabolism
- PMID: 35670584
- PMCID: PMC9238381
- DOI: 10.1128/aem.00507-22
Construction of a Rhodobacter sphaeroides Strain That Efficiently Produces Hydrogen Gas from Acetate without Poly(β-Hydroxybutyrate) Accumulation: Insight into the Role of PhaR in Acetate Metabolism
Abstract
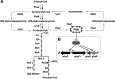
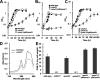
The purple nonsulfur phototrophic bacterium Rhodobacter sphaeroides produces hydrogen gas (H2) from acetate. An approach to improve the H2 production is preventing accumulation of an intracellular energy storage molecule known as poly(β-hydroxybutyrate) (PHB), which competes with H2 production for reducing power. However, disruption of PHB biosynthesis has been reported to severely impair the acetate assimilation depending on the genetic backgrounds and/or culture conditions. To solve this problem, we analyzed the relationship between PHB accumulation and acetate metabolism in R. sphaeroides. Gene deletion analyses based on the wild-type strain revealed that among the two polyhydroxyalkanoate synthase genes in the genome, phaC1, but not phaC2, is essential for PHB accumulation, and the phaC1 deletion mutant exhibited slow growth with acetate. On the other hand, a strain with the deletion of phaC1 together with phaR, which encodes a transcriptional regulator capable of sensing PHB accumulation, exhibited growth comparable to that of the wild-type strain despite no accumulation of PHB. These results suggest that PHB accumulation is required for normal growth with acetate by altering the expression of genes under the control of phaR. This hypothesis was supported by a transcriptome sequencing (RNA-seq) analysis revealing that phaR is involved in the regulation of the ethylmalonyl coenzyme A pathway for acetate assimilation. Consistent with these findings, deletion of phaC1 in a genetically engineered H2-producing strain resulted in lower H2 production from acetate due to growth defects, whereas deletion of phaR together with phaC1 restored growth with acetate and increased H2 production from acetate without PHB accumulation. IMPORTANCE This study provides a novel approach for increasing the yield of photofermentative H2 production from acetate by purple nonsulfur phototrophic bacteria. This study further suggests that polyhydroxyalkanoate is not only a storage substance for carbon and energy in bacteria, but may also act as a signaling molecule that mediates bacterial metabolic adaptations to specific environments. This notion will be helpful for understanding the physiology of polyhydroxyalkanoate-producing bacteria, as well as for their metabolic engineering via synthetic biology.
Keywords: PHB; PhaR; Rhodobacter; Rhodobacter sphaeroides; acetate; ethylmalonyl-CoA; hydrogen; hydrogen production; nitrogenase; polyhydroxyalkanoate; polyhydroxybutyrate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Khadka N, Milton RD, Shaw S, Lukoyanov D, Dean DR, Minteer SD, Raugei S, Hoffman BM, Seefeldt LC. 2017. Mechanism of nitrogenase H2 formation by metal-hydride protonation probed by mediated electrocatalysis and H/D isotope effects. J Am Chem Soc 139:13518–13524. doi:10.1021/jacs.7b07311. - DOI - PMC - PubMed
-
- Ghimire A, Frunzo L, Pirozzi F, Trably E, Escudie R, Lens PNL, Esposito G. 2015. A review on dark fermentative biohydrogen production from organic biomass: process parameters and use of by-products. Applied Energy 144:73–95. doi:10.1016/j.apenergy.2015.01.045. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

