A High-Throughput Yellow Fever Neutralization Assay
- PMID: 35670599
- PMCID: PMC9241659
- DOI: 10.1128/spectrum.02548-21
A High-Throughput Yellow Fever Neutralization Assay
Abstract
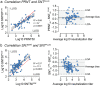
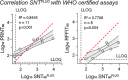
Quick and accurate detection of neutralizing antibodies (nAbs) against yellow fever is essential in serodiagnosis during outbreaks for surveillance and to evaluate vaccine efficacy in population-wide studies. All of this requires serological assays that can process a large number of samples in a highly standardized format. Albeit being laborious, time-consuming, and limited in throughput, the classical plaque reduction neutralization test (PRNT) is still considered the gold standard for the detection and quantification of nAbs due to its sensitivity and specificity. Here, we report the development of an alternative fluorescence-based serological assay (SNTFLUO) with an equally high sensitivity and specificity that is fit for high-throughput testing with the potential for automation. Finally, our novel SNTFLUO was cross-validated in several reference laboratories and against international WHO standards, showing its potential to be implemented in clinical use. SNTFLUO assays with similar performance are available for the Japanese encephalitis, Zika, and dengue viruses amenable to differential diagnostics. IMPORTANCE Fast and accurate detection of neutralizing antibodies (nAbs) against yellow fever virus (YFV) is key in yellow fever serodiagnosis, outbreak surveillance, and monitoring of vaccine efficacy. Although classical PRNT remains the gold standard for measuring YFV nAbs, this methodology suffers from inherent limitations such as low throughput and overall high labor intensity. We present a novel fluorescence-based serum neutralization test (SNTFLUO) with equally high sensitivity and specificity that is fit for processing a large number of samples in a highly standardized manner and has the potential to be implemented for clinical use. In addition, we present SNTFLUO assays with similar performance for Japanese encephalitis, Zika, and dengue viruses, opening new avenues for differential diagnostics.
Keywords: Japanese encephalitis virus; PRNT; Zika virus; dengue virus; high-throughput; neutralization assay; reporter virus; serodiagnosis; yellow fever virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization. 2019. Yellow fever. https://www.who.int/news-room/fact-sheets/detail/yellow-fever#:~:text=Ye.... Retrieved 2 November 2021.
-
- Lindenbach B, Thiel HJ, Rice CM. 2007. Flaviviridae: the viruses and their replication, p 1101–1151. In Knipe DM, Howley PM (ed), Fields virology, 5th ed. Wolters Kluwer Health, Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

