IL-10 promotes endothelial progenitor cell infiltration and wound healing via STAT3
- PMID: 35670763
- PMCID: PMC9796147
- DOI: 10.1096/fj.201901024RR
IL-10 promotes endothelial progenitor cell infiltration and wound healing via STAT3
Abstract
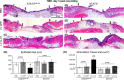
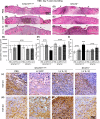
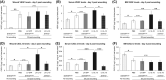
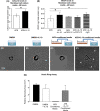
Endothelial progenitor cells (EPCs) contribute to de novo angiogenesis, tissue regeneration, and remodeling. Interleukin 10 (IL-10), an anti-inflammatory cytokine that primarily signals via STAT3, has been shown to drive EPC recruitment to injured tissues. Our previous work demonstrated that overexpression of IL-10 in dermal wounds promotes regenerative tissue repair via STAT3-dependent regulation of fibroblast-specific hyaluronan synthesis. However, IL-10's role and specific mode of action on EPC recruitment, particularly in dermal wound healing and neovascularization in both normal and diabetic wounds, remain to be defined. Therefore, inducible skin-specific STAT3 knockdown mice were studied to determine IL-10's impact on EPCs, dermal wound neovascularization and healing, and whether it is STAT3-dependent. We show that IL-10 overexpression significantly elevated EPC counts in the granulating wound bed, which was associated with robust capillary lumen density and enhanced re-epithelialization of both control and diabetic (db/db) wounds at day 7. We noted increased VEGF and high C-X-C motif chemokine 12 (CXCL12) levels in wounds and a favorable CXCL12 gradient at day 3 that may support EPC mobilization and infiltration from bone marrow to wounds, an effect that was abrogated in STAT3 knockdown wounds. These findings were supported in vitro. IL-10 promoted VEGF and CXCL12 synthesis in primary murine dermal fibroblasts, with blunted VEGF expression upon blocking CXCL12 in the media by antibody binding. IL-10-conditioned fibroblast media also significantly promoted endothelial sprouting and network formation. In conclusion, these studies demonstrate that overexpression of IL-10 in dermal wounds recruits EPCs and leads to increased vascular structures and faster re-epithelialization.
Keywords: IL-10; VEGF; angiogenesis; diabetes; endothelial progenitor cells; wound healing.
© 2022 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures



References
-
- Ribatti D, Vacca A, Nico B, Roncali L, Dammacco F. Postnatal vasculogenesis. Mech Dev. 2001;100(2):157‐163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

