Intestinal serotonergic system is modulated by Toll-like receptor 9
- PMID: 35670957
- PMCID: PMC9381617
- DOI: 10.1007/s13105-022-00897-2
Intestinal serotonergic system is modulated by Toll-like receptor 9
Abstract
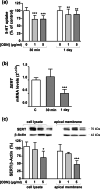
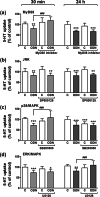
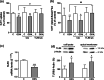
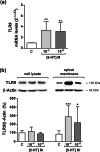
Intestinal serotonergic system is a key modulator of intestinal homeostasis; however, its regulation is still unclear. Toll-like receptor 9 (TLR9), an innate immune receptor, detects different external agents in the intestine, preserving intestinal integrity. Since little is known about TLR9 role in the intestine, our aim was to address the potential regulation between TLR9 and intestinal serotonergic system. Caco-2/TC7 cell line and intestinal tract of Tlr9-/- mice were used in this study. Serotonin uptake studies were performed, and molecular expression of different serotonergic components was analyzed by western blot and real-time PCR. Our results show that TLR9 activation inhibits serotonin transporter activity and expression, involving p38/MAPK and ERK/MAPK intracellular pathways, and reciprocally, serotonin increases TLR9 expression. Supporting this interaction, serotonin transporter, serotonin receptors and serotonin producer enzymes were found altered in intestinal tract of Tlr9-/- mice. We conclude that TLR9 could contribute to intestinal homeostasis by modulation of intestinal serotonergic system.
Keywords: Intestine; Microbiota; PRRs; SERT; Serotonin; TPH.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interests. The data that support the findings of this study are available from the corresponding author upon request.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

