Recurrent Plant-Specific Duplications of KNL2 and Its Conserved Function as a Kinetochore Assembly Factor
- PMID: 35671323
- PMCID: PMC9210943
- DOI: 10.1093/molbev/msac123
Recurrent Plant-Specific Duplications of KNL2 and Its Conserved Function as a Kinetochore Assembly Factor
Abstract
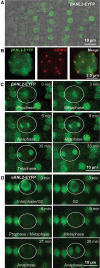
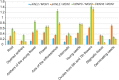
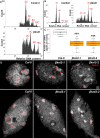
KINETOCHORE NULL2 (KNL2) plays key role in the recognition of centromeres and new CENH3 deposition. To gain insight into the origin and diversification of the KNL2 gene, we reconstructed its evolutionary history in the plant kingdom. Our results indicate that the KNL2 gene in plants underwent three independent ancient duplications in ferns, grasses and eudicots. Additionally, we demonstrated that previously unclassified KNL2 genes could be divided into two clades αKNL2 and βKNL2 in eudicots and γKNL2 and δKNL2 in grasses, respectively. KNL2s of all clades encode the conserved SANTA domain, but only the αKNL2 and γKNL2 groups additionally encode the CENPC-k motif. In the more numerous eudicot sequences, signatures of positive selection were found in both αKNL2 and βKNL2 clades, suggesting recent or ongoing adaptation. The confirmed centromeric localization of βKNL2 and mutant analysis suggests that it participates in loading of new CENH3, similarly to αKNL2. A high rate of seed abortion was found in heterozygous βKNL2 plants and the germinated homozygous mutants did not develop beyond the seedling stage. Taken together, our study provides a new understanding of the evolutionary diversification of the plant kinetochore assembly gene KNL2, and suggests that the plant-specific duplicated KNL2 genes are involved in centromere and/or kinetochore assembly for preserving genome stability.
Keywords: CENH3; KNL2; adaptive evolution; centromere; endopolyploidy; gene duplication; kinetochore.
© The Author(s) 2022. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Figures






References
-
- Ahmadli U, Sandmann M, Fuchs J, Lermontova I. 2022b. Immunolabeling of nuclei/chromosomes in Arabidopsis thaliana. In: Caillaud MC, editor. Plant cell division. Methods in molecular biology, vol. 2382. New York: (NY: ): Humana. p. 19–28. - PubMed
-
- Bailey TL, Gribskov M. 1998. Combining evidence using p-values: application to sequence homology searches. Bioinformatics. 14(1):48–54. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

