Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial
- PMID: 35671327
- PMCID: PMC9173627
- DOI: 10.1371/journal.pmed.1003998
Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial
Abstract
Background: STAMPEDE has previously reported that radiotherapy (RT) to the prostate improved overall survival (OS) for patients with newly diagnosed prostate cancer with low metastatic burden, but not those with high-burden disease. In this final analysis, we report long-term findings on the primary outcome measure of OS and on the secondary outcome measures of symptomatic local events, RT toxicity events, and quality of life (QoL).
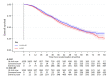
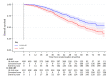
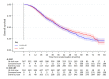
Methods and findings: Patients were randomised at secondary care sites in the United Kingdom and Switzerland between January 2013 and September 2016, with 1:1 stratified allocation: 1,029 to standard of care (SOC) and 1,032 to SOC+RT. No masking of the treatment allocation was employed. A total of 1,939 had metastatic burden classifiable, with 42% low burden and 58% high burden, balanced by treatment allocation. Intention-to-treat (ITT) analyses used Cox regression and flexible parametric models (FPMs), adjusted for stratification factors age, nodal involvement, the World Health Organization (WHO) performance status, regular aspirin or nonsteroidal anti-inflammatory drug (NSAID) use, and planned docetaxel use. QoL in the first 2 years on trial was assessed using prospectively collected patient responses to QLQ-30 questionnaire. Patients were followed for a median of 61.3 months. Prostate RT improved OS in patients with low, but not high, metastatic burden (respectively: 202 deaths in SOC versus 156 in SOC+RT, hazard ratio (HR) = 0·64, 95% CI 0.52, 0.79, p < 0.001; 375 SOC versus 386 SOC+RT, HR = 1.11, 95% CI 0.96, 1.28, p = 0·164; interaction p < 0.001). No evidence of difference in time to symptomatic local events was found. There was no evidence of difference in Global QoL or QLQ-30 Summary Score. Long-term urinary toxicity of grade 3 or worse was reported for 10 SOC and 10 SOC+RT; long-term bowel toxicity of grade 3 or worse was reported for 15 and 11, respectively.
Conclusions: Prostate RT improves OS, without detriment in QoL, in men with low-burden, newly diagnosed, metastatic prostate cancer, indicating that it should be recommended as a SOC.
Trial registration: ClinicalTrials.gov NCT00268476, ISRCTN.com ISRCTN78818544.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: CCP reports personal fees from Bayer, personal fees from Janssen, personal fees from Clarity Pharmaceuticals, personal fees from Myovant, personal fees from ITM Oncologics, outside the submitted work NDJ received research funding to the institution from Astellas, Astra Zeneca &Janssen; receipt of honoraria/fees on the advisory board for Astra Zenenca, Clovis, Janssen, Merck, Novartis & Sanofi; received fees as a speaker for Bayer & Novartis NWC received honoraria from Astellas & Janssen; took a consulting/advisory role for Astellas, Janssen, Ferring, Bayer & Sanofi; was paid speakers fees b Janssen & Astellas; received funding for the institution from Astra Zeneca; received meeting and travel expenses from Janssen, Astellas, Sanofi, Astra Zeneca, Ferring & Ipsen GA reports personal fees from Sanofi Aventis, during the conduct of the study; personal fees and non-financial support from Astellas, personal fees and non-financial support from Medivation, personal fees from Novartis, personal fees from Millennium Pharmaceuticals, personal fees and non-financial support from Abbott Laboratories, personal fees and non-financial support from Essa Pharmaceuticals, personal fees and non-financial support from Bayer Healthcare Pharmaceuticals, personal fees from Takeda, grants from AstraZeneca, grants from Arno Therapeutics, grants from Innocrin Pharma, grants, personal fees and non-financial support from Janssen, personal fees from Veridex, personal fees and non-financial support from Roche/Ventana, personal fees and non-financial support from Pfizer, personal fees from The Institute of Cancer Research (ICR), outside the submitted work; and The Institute of Cancer Research (ICR) receives royalty income from abiraterone I receive a share of this income through the ICR’s Rewards to Discoverers Scheme SC received consulting fees from Telix, remedy & Huma; received payment for speaker fees and/or manuscript writing and/or educational events from Astra Zeneca, Novartis/AAA, Clovis, Janssen, Bayer, Pfizer, Beigene & Astellas; they were a member of the data safety monitoring/advisory board for Astra Zeneca, Novartis/AAA, Clovis, Janssen, Bayer, Pfizer, Beigene & Astellas DPD received payment to the institution from C33589/A19727 Advances in Physics for Precision Radiotherapy; previous employer, The Institute of Cancer Research receives loyalty income from abiraterone, receives personal share of this income through ICR’s Rewards to Discoverer’s Scheme; honoraria for consultancy from Janssen; EP1933709B1 – Location and Stabilisation Device., European patent issued, Pending in Canada and India SG reports personal fees from Orion, personal fees from Janssen Cilag, personal fees from ProteoMedix, personal fees from Amgen, personal fees from MSD, other from Tolero Pharmaceuticals, other from Astellas Pharma, other from Janssen, other from MSD Merck Sharp&Dome, other from Bayer, other from Roche, other from Pfizer, other from Telixpharma, other from Amgen, other from Bristol-Myers Squibb, other from AAA International SA, other from Orion, other from Silvio Grasso Consulting, from Tolremo, outside the submitted work; In addition, Gillessen has a patent WO2009138392 issued and Menarini Silicon Biosystems (Advisory Board 2019) - not compensated Aranda (Advisory Board 2019) - not compensated RJJ received research funding to the institution from Bayer, Astellas & Pfizer; received honoraria on the advisory board for Janssen, Astellas, Bayer, Pfizer; received speaker fees from Janssen, Astellas, Bayer & Pfizer REL received an institutional grant from the MRC CG received research funding to the institution from Janssen, Clovis Oncology, Sanofi, Astellas, Medical Research Council & Cancer Research UK DF received speaker fees and/or manuscript writing and/or educational events from BMS, IPSEN, EUSA, Pfizer, ESAI; they received travel expenses from Janssen & IPSEN MDM is an advisory board member for Endocyte & Clovis AB received payment for lecture/presentation/speaker bureau/manuscript writing or educational event from Boston Scientific AJB received speaker fees and travel support from Janssen DF received payment for lectures for Janssen, Pfizer & BMS; support for attending conferences/meetings from Genisiscare & BMS AMH received research grants from CRUK and NIHR; support attending meetings from the European Association of Urologists; is a member of the European Association of Urologists & the Prostate Cancer Guidelines Group MK received travel, accommodation and conference fees as expenses from Bayer, travel and accommodation fees for Prostate cancer summits from Janssen AL received expenses for attending meetings and/or travel from Astellas, Bayer, BMS & MSD JMOS received speaker fees from AAA, Astellas, Bayer, Janssen, Novartis, Sanofi and participated as an advisory board member and/or member of the data safety monitoring board for AAA, Astellas, Bayer, Janssen, Novartis & Sanofi NNS received travel/meeting payments from Janssen JT received support for conference attendance from Janssen, Roche & Bayer; participated on the advisory board for Astra Zeneca, Astellas & Bayer MKBP received research funding to the Unit he directs from Acoria Pvt Ltd, Akagera, Amgen, Aspirin Foundation, Astellas, AstraZeneca, Baxter, Bayer, BMS US, Bri-Bio, Cepheid, Cipla, Clovis Inc, CSL Behring, Eli-Lilly, Emergent Biosolutions, Gilead Sciences, GlaxoSmithKline, Grifols, Janssen Products LP, Janssen-Cilag, Johnson & Johnson, Micronoma, Modus Theraputics, Mylan, Novartis, Pfizer, Sanofi, Serum Institute of India, Shionogi, Synteny Biotechnology, Takeda, Tibotec, Transgene, ViiV Healthcare, Virco and Xenothera MRS received research funding to the institution from Astellas, Clovis, Janssen, Novartis, Pfizer, Sanofi-Aventis; received speaker fees from Lilly Oncology & Janssen; independent member of data monitoring committees. All other authors have nothing to declare.
Figures
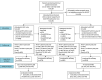

Comment in
-
Urological Oncology: Prostate Cancer.J Urol. 2022 Oct;208(4):931-934. doi: 10.1097/JU.0000000000002870. Epub 2022 Aug 16. J Urol. 2022. PMID: 35973210 No abstract available.
References
-
- Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al.. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353–66. Epub 2018/10/26. doi: 10.1016/S0140-6736(18)32486-3 ; PubMed Central PMCID: PMC6269599. - DOI - PMC - PubMed

