Homogeneous Organic Electron Donors in Nickel-Catalyzed Reductive Transformations
- PMID: 35671350
- PMCID: PMC9335070
- DOI: 10.1021/acs.joc.2c00462
Homogeneous Organic Electron Donors in Nickel-Catalyzed Reductive Transformations
Abstract
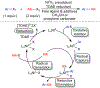
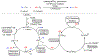
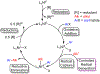
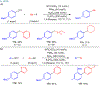
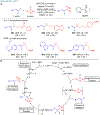
Many contemporary organic transformations, such as Ni-catalyzed cross-electrophile coupling (XEC), require a reductant. Typically, heterogeneous reductants, such as Zn0 or Mn0, are used as the electron source in these reactions. Although heterogeneous reductants are highly practical for preparative-scale batch reactions, they can lead to complications in performing reactions on process scale and are not easily compatible with modern applications, such as flow chemistry. In principle, homogeneous organic reductants can address some of the challenges associated with heterogeneous reductants and also provide greater control of the reductant strength, which can lead to new reactivity. Nevertheless, homogeneous organic reductants have rarely been used in XEC. In this Perspective, we summarize recent progress in the use of homogeneous organic electron donors in Ni-catalyzed XEC and related reactions, discuss potential synthetic and mechanistic benefits, describe the limitations that inhibit their implementation, and outline challenges that need to be solved in order for homogeneous organic reductants to be widely utilized in synthetic chemistry. Although our focus is on XEC, our discussion of the strengths and weaknesses of different methods for introducing electrons is general to other reductive transformations.
Conflict of interest statement
Competing Financial Interests
The authors declare no competing financial interests.
Figures
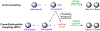
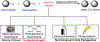

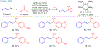

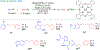









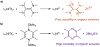

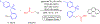

Similar articles
-
Tunable and Practical Homogeneous Organic Reductants for Cross-Electrophile Coupling.J Am Chem Soc. 2021 Dec 15;143(49):21024-21036. doi: 10.1021/jacs.1c10932. Epub 2021 Nov 30. J Am Chem Soc. 2021. PMID: 34846142 Free PMC article.
-
Insights into Recent Nickel-Catalyzed Reductive and Redox C-C Coupling of Electrophiles, C(sp3)-H Bonds and Alkenes.Acc Chem Res. 2024 Apr 16;57(8):1149-1162. doi: 10.1021/acs.accounts.3c00810. Epub 2024 Mar 28. Acc Chem Res. 2024. PMID: 38547518
-
Zinc and manganese redox potentials in organic solvents and their influence on nickel-catalysed cross-electrophile coupling.Nat Chem. 2024 Dec;16(12):2036-2043. doi: 10.1038/s41557-024-01627-5. Epub 2024 Sep 6. Nat Chem. 2024. PMID: 39242931 Free PMC article.
-
Gold-Catalyzed Cross-Coupling Reactions: An Overview of Design Strategies, Mechanistic Studies, and Applications.Chemistry. 2020 Feb 3;26(7):1442-1487. doi: 10.1002/chem.201903377. Epub 2019 Dec 10. Chemistry. 2020. PMID: 31657487 Review.
-
Cross-Electrophile Coupling: Principles, Methods, and Applications in Synthesis.Chem Rev. 2024 Dec 11;124(23):13397-13569. doi: 10.1021/acs.chemrev.4c00524. Epub 2024 Nov 26. Chem Rev. 2024. PMID: 39591522 Free PMC article. Review.
Cited by
-
Ligand-Metal Cooperation Enables Net Ring-Opening C-C Activation / Difunctionalization of Cyclopropyl Ketones.ACS Catal. 2023 Sep 1;13(17):11277-11290. doi: 10.1021/acscatal.3c02643. Epub 2023 Aug 11. ACS Catal. 2023. PMID: 39386022 Free PMC article.
-
Nickel-Catalyzed Enantioselective Electrochemical Reductive Cross-Coupling of Aryl Aziridines with Alkenyl Bromides.J Am Chem Soc. 2023 Mar 22;145(11):6270-6279. doi: 10.1021/jacs.2c12869. Epub 2023 Mar 7. J Am Chem Soc. 2023. PMID: 36881734 Free PMC article.
-
Bis(pinacolato)diboron-Enabled Ni-Catalyzed Reductive Arylation/Vinylation of Alkyl Electrophiles.Adv Sci (Weinh). 2024 Aug;11(31):e2404301. doi: 10.1002/advs.202404301. Epub 2024 Jun 17. Adv Sci (Weinh). 2024. PMID: 38887210 Free PMC article.
-
Aryl halide cross-coupling via formate-mediated transfer hydrogenation.Nat Chem. 2025 May;17(5):710-718. doi: 10.1038/s41557-024-01729-0. Epub 2025 Mar 11. Nat Chem. 2025. PMID: 40069564 Free PMC article.
-
Cyclopropanation with Non-Stabilized Carbenes via Ketyl Radicals.J Am Chem Soc. 2024 Aug 28;146(34):24009-24015. doi: 10.1021/jacs.4c07388. Epub 2024 Jul 24. J Am Chem Soc. 2024. PMID: 39049431 Free PMC article.
References
-
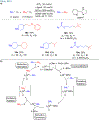
- In this work, we define a homogeneous electron donor as a molecule that transfers electrons to a catalyst or substrate in the same phase (for example liquid to liquid) and does not require activation by light.
-
- Liu J; Lu L; Wood D; Lin S New Redox Strategies in Organic Synthesis by Means of Electrochemistry and Photochemistry. ACS Cent. Sci 2020, 6, 1317–1340; - PMC - PubMed
- Rein J; Annand JR; Wismer MK; Fu J; Siu JC; Klapars A; Strotman NA; Kalyani D; Lehnherr D; Lin S Unlocking the Potential of High-Throughput Experimentation for Electrochemistry with a Standardized Microscale Reactor. ACS Cent. Sci 2021, 7, 1347–1355; - PMC - PubMed
- Hardwick T; Ahmed N C–H Functionalization via Electrophotocatalysis and Photoelectrochemistry: Complementary Synthetic Approach. ACS Sustain. Chem. Eng 2021, 9, 4324–4340;
- Beutner GL; Simmons EM; Ayers S; Bemis CY; Goldfogel MJ; Joe CL; Marshall J; Wisniewski SR A Process Chemistry Benchmark for sp2–sp3 Cross Couplings. J. Org. Chem 2021, 86, 10380–10396; - PubMed
- Buglioni L; Raymenants F; Slattery A; Zondag SDA; Noël T Technological Innovations in Photochemistry for Organic Synthesis: Flow Chemistry, High-Throughput Experimentation, Scale-up, and Photoelectrochemistry. Chem. Rev 2022, 122, 2752–2906; - PMC - PubMed
- Tay NES; Lehnherr D; Rovis T Photons or Electrons? A Critical Comparison of Electrochemistry and Photoredox Catalysis for Organic Synthesis. Chem. Rev 2022, 122, 2487–2649. - PMC - PubMed
-
- Everson DA; Weix DJ Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem 2014, 79, 4793–4798; - PMC - PubMed
- Knappke CEI; Grupe S; Gärtner D; Corpet M; Gosmini C; Jacobi von Wangelin A Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. Eur. J 2014, 20, 6828–6842; - PubMed
- Moragas T; Correa A; Martin R Metal-Catalyzed Reductive Coupling Reactions of Organic Halides with Carbonyl-Type Compounds. Chem. Eur. J 2014, 20, 8242–8258; - PubMed
- Weix DJ Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res 2015, 48, 1767–1775; - PMC - PubMed
- Gu J; Wang X; Xue W; Gong H Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Other Electrophiles: Concept and Mechanistic Considerations. Org. Chem. Front 2015, 2, 1411–1421;
- Richmond E; Moran J Recent Advances in Nickel Catalysis Enabled by Stoichiometric Metallic Reducing Agents. Synthesis 2017, 50, 499–513;
- Campeau L-C; Hazari N Cross-Coupling and Related Reactions: Connecting Past Success to the Development of New Reactions for the Future. Organometallics 2019, 38, 3–35; - PMC - PubMed
- Liu J; Ye Y; Sessler JL; Gong H Cross-Electrophile Couplings of Activated and Sterically Hindered Halides and Alcohol Derivatives. Acc. Chem. Res 2020, 53, 1833–1845; - PubMed
- Poremba KE; Dibrell SE; Reisman SE Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions. ACS Catal 2020, 10, 8237–8246. - PMC - PubMed
-
-
Although not the focus of this review, Co-catalyzed systems for XEC have also been developed and are important. To date these almost exclusively use heterogeneous reductants. For leading references see:
- Dorval C; Gosmini C In Cobalt Catalysis in Organic Synthesis. Hapke M, Hilt G, Eds.; Wiley-VCH: Weinheim, 2020; pp 163–205;
- Cai Y; Qian X; Gosmini C Cobalt-Catalyzed C−C Homocoupling. Adv. Synth. Catal 2016, 358, 2427–2430;
- Pal S; Chowdhury S; Rozwadowski E; Auffrant A; Gosmini C Cobalt-Catalyzed Reductive Cross-Coupling Between Benzyl Chlorides and Aryl Halides. Adv. Synth. Catal 2016, 358, 2431–2435.
- Cai Y; Benischke AD; Knochel P; Gosmini C Cobalt-Catalyzed Reductive Cross-Coupling Between Styryl and Benzyl Halides. Chem. Eur. J 2017, 23, 250–253. - PubMed
- Reddy BRP; Chowdhury S; Auffrant A; Gosmini C Cobalt-Catalyzed Formation of Functionalized Diarylmethanes from Benzylmesylates and Aryl Halides. Adv. Synth. Catal 2018, 360, 3026–3029.
- Dorval C; Tricoire M; Begouin J-M; Gandon V; Gosmini C Cobalt-Catalyzed C(sp2)–CN Bond Activation: Cross-Electrophile Coupling for Biaryl Formation and Mechanistic Insight. ACS Catal 2020, 10, 12819–12827.
-
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

