Outcomes following treatment for ADA-deficient severe combined immunodeficiency: a report from the PIDTC
- PMID: 35671392
- PMCID: PMC9389638
- DOI: 10.1182/blood.2022016196
Outcomes following treatment for ADA-deficient severe combined immunodeficiency: a report from the PIDTC
Abstract
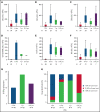
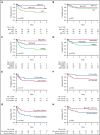
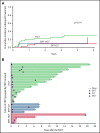
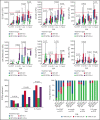
Adenosine deaminase (ADA) deficiency causes ∼13% of cases of severe combined immune deficiency (SCID). Treatments include enzyme replacement therapy (ERT), hematopoietic cell transplant (HCT), and gene therapy (GT). We evaluated 131 patients with ADA-SCID diagnosed between 1982 and 2017 who were enrolled in the Primary Immune Deficiency Treatment Consortium SCID studies. Baseline clinical, immunologic, genetic characteristics, and treatment outcomes were analyzed. First definitive cellular therapy (FDCT) included 56 receiving HCT without preceding ERT (HCT); 31 HCT preceded by ERT (ERT-HCT); and 33 GT preceded by ERT (ERT-GT). Five-year event-free survival (EFS, alive, no need for further ERT or cellular therapy) was 49.5% (HCT), 73% (ERT-HCT), and 75.3% (ERT-GT; P < .01). Overall survival (OS) at 5 years after FDCT was 72.5% (HCT), 79.6% (ERT-HCT), and 100% (ERT-GT; P = .01). Five-year OS was superior for patients undergoing HCT at <3.5 months of age (91.6% vs 68% if ≥3.5 months, P = .02). Active infection at the time of HCT (regardless of ERT) decreased 5-year EFS (33.1% vs 68.2%, P < .01) and OS (64.7% vs 82.3%, P = .02). Five-year EFS (90.5%) and OS (100%) were best for matched sibling and matched family donors (MSD/MFD). For patients treated after the year 2000 and without active infection at the time of FDCT, no difference in 5-year EFS or OS was found between HCT using a variety of transplant approaches and ERT-GT. This suggests alternative donor HCT may be considered when MSD/MFD HCT and GT are not available, particularly when newborn screening identifies patients with ADA-SCID soon after birth and before the onset of infections. This trial was registered at www.clinicaltrials.gov as #NCT01186913 and #NCT01346150.
Figures


Comment in
-
Four decades of progress.Blood. 2022 Aug 18;140(7):665-666. doi: 10.1182/blood.2022017211. Blood. 2022. PMID: 35980683 No abstract available.
References
-
- Giblett ER, Anderson JE, Cohen F, Pollara B, Meuwissen HJ. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972; 2(7786):1067-1069. - PubMed
-
- Grunebaum E, Cohen A, Roifman CM. Recent advances in understanding and managing adenosine deaminase and purine nucleoside phosphorylase deficiencies. Curr Opin Allergy Clin Immunol. 2013;13(6): 630-638. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

