Hepatitis E virus infects brain microvascular endothelial cells, crosses the blood-brain barrier, and invades the central nervous system
- PMID: 35671427
- PMCID: PMC9214495
- DOI: 10.1073/pnas.2201862119
Hepatitis E virus infects brain microvascular endothelial cells, crosses the blood-brain barrier, and invades the central nervous system
Abstract
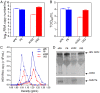
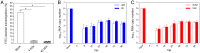
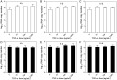
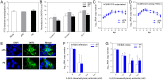
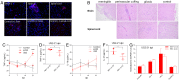
Hepatitis E virus (HEV) is an important but understudied zoonotic virus causing both acute and chronic viral hepatitis. A proportion of HEV-infected individuals also developed neurological diseases such as Guillain-Barré syndrome, neuralgic amyotrophy, encephalitis, and myelitis, although the mechanism remains unknown. In this study, by using an in vitro blood-brain barrier (BBB) model, we first investigated whether HEV can cross the BBB and whether the quasi-enveloped HEV virions are more permissible to the BBB than the nonenveloped virions. We found that both quasi-enveloped and nonenveloped HEVs can similarly cross the BBB and that addition of proinflammatory cytokine tumor necrosis factor alpha (TNF-α) has no significant effect on the ability of HEV to cross the BBB in vitro. To explore the possible mechanism of HEV entry across the BBB, we tested the susceptibility of human brain microvascular endothelial cells lining the BBB to HEV infection and showed that brain microvascular endothelial cells support productive HEV infection. To further confirm the in vitro observation, we conducted an experimental HEV infection study in pigs and showed that both quasi-enveloped and nonenveloped HEVs invade the central nervous system (CNS) in pigs, as HEV RNA was detected in the brain and spinal cord of infected pigs. The HEV-infected pigs with detectable viral RNA in CNS tissues had histological lesions in brain and spinal cord and significantly higher levels of proinflammatory cytokines TNF-α and interleukin 18 than the HEV-infected pigs without detectable viral RNA in CNS tissues. The findings suggest a potential mechanism of HEV-associated neuroinvasion.
Keywords: blood–brain barrier (BBB); brain microvascular endothelial cells; central nervous system (CNS); hepatitis E virus (HEV); neurological disorder.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Rein D. B., Stevens G. A., Theaker J., Wittenborn J. S., Wiersma S. T., The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 55, 988–997 (2012). - PubMed
-
- Geng Y., et al. , High seroprevalence of hepatitis E virus in rabbit slaughterhouse workers. Transbound. Emerg. Dis. 66, 1085–1089 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

