Vasculopathy and Increased Vascular Congestion in Fatal COVID-19 and Acute Respiratory Distress Syndrome
- PMID: 35671465
- PMCID: PMC9799276
- DOI: 10.1164/rccm.202109-2150OC
Vasculopathy and Increased Vascular Congestion in Fatal COVID-19 and Acute Respiratory Distress Syndrome
Abstract
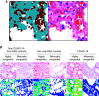
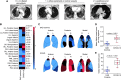
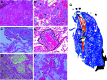
Rationale: The leading cause of death in coronavirus disease 2019 (COVID-19) is severe pneumonia, with many patients developing acute respiratory distress syndrome (ARDS) and diffuse alveolar damage (DAD). Whether DAD in fatal COVID-19 is distinct from other causes of DAD remains unknown. Objective: To compare lung parenchymal and vascular alterations between patients with fatal COVID-19 pneumonia and other DAD-causing etiologies using a multidimensional approach. Methods: This autopsy cohort consisted of consecutive patients with COVID-19 pneumonia (n = 20) and with respiratory failure and histologic DAD (n = 21; non-COVID-19 viral and nonviral etiologies). Premortem chest computed tomography (CT) scans were evaluated for vascular changes. Postmortem lung tissues were compared using histopathological and computational analyses. Machine-learning-derived morphometric analysis of the microvasculature was performed, with a random forest classifier quantifying vascular congestion (CVasc) in different microscopic compartments. Respiratory mechanics and gas-exchange parameters were evaluated longitudinally in patients with ARDS. Measurements and Main Results: In premortem CT, patients with COVID-19 showed more dilated vasculature when all lung segments were evaluated (P = 0.001) compared with controls with DAD. Histopathology revealed vasculopathic changes, including hemangiomatosis-like changes (P = 0.043), thromboemboli (P = 0.0038), pulmonary infarcts (P = 0.047), and perivascular inflammation (P < 0.001). Generalized estimating equations revealed significant regional differences in the lung microarchitecture among all DAD-causing entities. COVID-19 showed a larger overall CVasc range (P = 0.002). Alveolar-septal congestion was associated with a significantly shorter time to death from symptom onset (P = 0.03), length of hospital stay (P = 0.02), and increased ventilatory ratio [an estimate for pulmonary dead space fraction (Vd); p = 0.043] in all cases of ARDS. Conclusions: Severe COVID-19 pneumonia is characterized by significant vasculopathy and aberrant alveolar-septal congestion. Our findings also highlight the role that vascular alterations may play in Vd and clinical outcomes in ARDS in general.
Keywords: ARDS; COVID-19; vascular congestion; vasculopathy; ventilatory ratio.
Figures



Comment in
-
Pulmonary Vasculopathy in COVID-19 Acute Respiratory Distress Syndrome: A Step Closer to the Full Picture.Am J Respir Crit Care Med. 2022 Oct 1;206(7):809-810. doi: 10.1164/rccm.202205-1019ED. Am J Respir Crit Care Med. 2022. PMID: 35675564 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

