Toward a Biomimetic Neural Circuit Model of Sensory-Motor Processing
- PMID: 35671470
- PMCID: PMC9971833
- DOI: 10.1162/neco_a_01516
Toward a Biomimetic Neural Circuit Model of Sensory-Motor Processing
Abstract
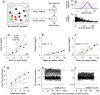
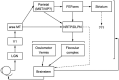
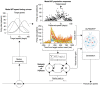
Computational models have been a mainstay of research on smooth pursuit eye movements in monkeys. Pursuit is a sensory-motor system that is driven by the visual motion of small targets. It creates a smooth eye movement that accelerates up to target speed and tracks the moving target essentially perfectly. In this review of my laboratory's research, I trace the development of computational models of pursuit eye movements from the early control-theory models to the most recent neural circuit models. I outline a combined experimental and computational plan to move the models to the next level. Finally, I explain why research on nonhuman primates is so critical to the development of the neural circuit models I think we need.
© 2022 Massachusetts Institute of Technology.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

