The secretome of irradiated peripheral blood mononuclear cells attenuates activation of mast cells and basophils
- PMID: 35671621
- PMCID: PMC9168057
- DOI: 10.1016/j.ebiom.2022.104093
The secretome of irradiated peripheral blood mononuclear cells attenuates activation of mast cells and basophils
Abstract
Background: IgE-mediated hypersensitivity is becoming increasingly prevalent and activation of mast cells and basophils represent key events in the pathophysiology of allergy. We have previously reported that the secretome of γ-irradiated peripheral blood mononuclear cells (PBMCsec) exerts beneficial anti-inflammatory effects. Yet, its ability to alleviate allergic symptoms has not been investigated so far.
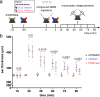
Methods: Several experimental in vitro and in vivo models have been used in this basic research study. A murine ear swelling model was used to study the effects of PBMCsec on 48/80-induced mast cell degranulation in vivo. The transcriptional profile of murine mast cells was analysed by single cell RNA sequencing (scRNAseq). Mast cell activation was studied in vitro using primary skin mast cells. Basophils from individuals allergic to birch pollens were used to investigate basophile activation by allergens. Transcriptomic and lipidomic analyses were used to identify mRNA expression and lipid species present in PBMCsec, respectively.
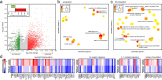
Findings: Topical application of PBMCsec on mouse ears (C57BL/6) significantly reduced tissue swelling following intradermal injection of compound 48/80, an inducer of mast cell degranulation. Single cell RNA sequencing of PBMCsec-treated murine dermal mast cells (Balb/c) revealed a downregulation of genes involved in immune cell degranulation and Fc-receptor signalling. In addition, treatment of primary human dermal mast cells with PBMCsec strongly inhibited compound 48/80- and α-IgE-induced mediator release in vitro. Furthermore, PBMCsec remarkably attenuated allergen driven activation of basophils from allergic individuals. Transcriptomic analysis of these basophils showed that PBMCsec downregulated a distinct gene battery involved in immune cell degranulation and Fc-receptor signalling, corroborating results obtained from dermal mast cells. Finally, we identified the lipid fraction of PBMCsec as the major active ingredient involved in effector cell inhibition.
Interpretation: Collectively, our data demonstrate that PBMCsec is able to reduce activation of mast cells and basophils, encouraging further studies on the potential use of PBMCsec for treating allergy.
Funding: Austrian Research Promotion Agency (852748 and 862068, 2015-2019), Vienna Business Agency (2343727, 2018-2020), Aposcience AG, Austrian Federal Ministry of Education, Science and Research (SPA06/055), Danube Allergy Research Cluster, Austrian Science Fund (I4437 and P32953).
Keywords: Anti-allergic therapeutic secretome; Basophil activation; Birch pollen allergy; Mast cell degranulation.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The Medical University of Vienna has claimed financial interest. HJA holds patents related to this work (WO2010079086A1; WO2010070105A1; EP3502692A1; WO2021130305A1). MM hold a patent related to this work (WO2021130305A1). ML, DC, VV, AG, MD, KK, DB, AP, and HJA are affiliated with the company Aposcience AG, a manufacturer of PBMCsec. All other authors declare no potential conflicts of interest.
Figures




Similar articles
-
The Secretome of Irradiated Peripheral Mononuclear Cells Attenuates Hypertrophic Skin Scarring.Pharmaceutics. 2023 Mar 25;15(4):1065. doi: 10.3390/pharmaceutics15041065. Pharmaceutics. 2023. PMID: 37111549 Free PMC article.
-
Therapeutic potential of lipids obtained from γ-irradiated PBMCs in dendritic cell-mediated skin inflammation.EBioMedicine. 2020 May;55:102774. doi: 10.1016/j.ebiom.2020.102774. Epub 2020 May 8. EBioMedicine. 2020. PMID: 32403085 Free PMC article.
-
Allergen-Specific IgA Antibodies Block IgE-Mediated Activation of Mast Cells and Basophils.Front Immunol. 2022 Jul 5;13:881655. doi: 10.3389/fimmu.2022.881655. eCollection 2022. Front Immunol. 2022. PMID: 35865546 Free PMC article.
-
IgE and IgG Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy.Front Immunol. 2020 Dec 11;11:603050. doi: 10.3389/fimmu.2020.603050. eCollection 2020. Front Immunol. 2020. PMID: 33362785 Free PMC article. Review.
-
News in Cellular Allergology: A Review of the Human Mast Cell and Basophil Granulocyte Literature from January 2013 to May 2015.Int Arch Allergy Immunol. 2015;168(4):253-62. doi: 10.1159/000443960. Epub 2016 Feb 20. Int Arch Allergy Immunol. 2015. PMID: 26895271 Review.
Cited by
-
The Effect of Paracrine Factors Released by Irradiated Peripheral Blood Mononuclear Cells on Neutrophil Extracellular Trap Formation.Antioxidants (Basel). 2022 Aug 11;11(8):1559. doi: 10.3390/antiox11081559. Antioxidants (Basel). 2022. PMID: 36009277 Free PMC article.
-
Lipids of Platelet-Rich Fibrin Reduce the Inflammatory Response in Mesenchymal Cells and Macrophages.Cells. 2023 Feb 16;12(4):634. doi: 10.3390/cells12040634. Cells. 2023. PMID: 36831301 Free PMC article.
-
Paracrine Factors of Stressed Peripheral Blood Mononuclear Cells Activate Proangiogenic and Anti-Proteolytic Processes in Whole Blood Cells and Protect the Endothelial Barrier.Pharmaceutics. 2022 Jul 30;14(8):1600. doi: 10.3390/pharmaceutics14081600. Pharmaceutics. 2022. PMID: 36015226 Free PMC article.
-
The Secretome of Irradiated Peripheral Mononuclear Cells Attenuates Hypertrophic Skin Scarring.Pharmaceutics. 2023 Mar 25;15(4):1065. doi: 10.3390/pharmaceutics15041065. Pharmaceutics. 2023. PMID: 37111549 Free PMC article.
References
-
- Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. - PubMed
-
- In: Oria MP, Stallings VA, editors. Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management, and Public Policy. Washington (DC) 2016. - PubMed
-
- Biedermann T, Winther L, Till SJ, Panzner P, Knulst A, Valovirta E. Birch pollen allergy in Europe. Allergy. 2019;74(7):1237–1248. - PubMed
-
- Stemeseder T, Klinglmayr E, Moser S, et al. Cross-sectional study on allergic sensitization of Austrian adolescents using molecule-based IgE profiling. Allergy. 2017;72(5):754–763. - PubMed
-
- Moverare R, Westritschnig K, Svensson M, et al. Different IgE reactivity profiles in birch pollen-sensitive patients from six European populations revealed by recombinant allergens: an imprint of local sensitization. Int Arch Allergy Immunol. 2002;128(4):325–335. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

