Parsing the role of NSP1 in SARS-CoV-2 infection
- PMID: 35671758
- PMCID: PMC9133101
- DOI: 10.1016/j.celrep.2022.110954
Parsing the role of NSP1 in SARS-CoV-2 infection
Abstract
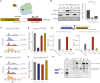
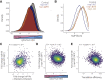
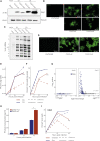
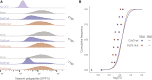
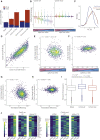
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leads to shutoff of protein synthesis, and nsp1, a central shutoff factor in coronaviruses, inhibits cellular mRNA translation. However, the diverse molecular mechanisms employed by nsp1 as well as its functional importance are unresolved. By overexpressing various nsp1 mutants and generating a SARS-CoV-2 mutant, we show that nsp1, through inhibition of translation and induction of mRNA degradation, targets translated cellular mRNA and is the main driver of host shutoff during infection. The propagation of nsp1 mutant virus is inhibited exclusively in cells with intact interferon (IFN) pathway as well as in vivo, in hamsters, and this attenuation is associated with stronger induction of type I IFN response. Therefore, although nsp1's shutoff activity is broad, it plays an essential role, specifically in counteracting the IFN response. Overall, our results reveal the multifaceted approach nsp1 uses to shut off cellular protein synthesis and uncover nsp1's explicit role in blocking the IFN response.
Keywords: CP: Microbiology; Coronaviruses; Host shutoff; Interferon; Nsp1; RNA; SARS-CoV-2; Translation regulation.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Update of
-
Parsing the role of NSP1 in SARS-CoV-2 infection.bioRxiv [Preprint]. 2022 Mar 16:2022.03.14.484208. doi: 10.1101/2022.03.14.484208. bioRxiv. 2022. Update in: Cell Rep. 2022 Jun 14;39(11):110954. doi: 10.1016/j.celrep.2022.110954. PMID: 35313595 Free PMC article. Updated. Preprint.
References
-
- Addetia A., Lieberman N.A.P., Phung Q., Hsiang T.-Y., Xie H., Roychoudhury P., Shrestha L., Loprieno M.A., Huang M.-L., Gale M., Jr., et al. SARS-CoV-2 ORF6 disrupts bidirectional nucleocytoplasmic transport through interactions with Rae1 and Nup98. mBio. 2021;12:e00065-21. doi: 10.1128/mbio.00065-21. - DOI - PMC - PubMed
-
- Banerjee A.K., Blanco M.R., Bruce E.A., Honson D.D., Chen L.M., Chow A., Bhat P., Ollikainen N., Quinodoz S.A., Loney C., et al. SARS-CoV-2 disrupts splicing, translation, and protein trafficking to suppress host defenses. Cell. 2020;183:1325–1339.e21. doi: 10.1016/j.cell.2020.10.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

