Development of an automated screen for Kv7.2 potassium channels and discovery of a new agonist chemotype
- PMID: 35671848
- PMCID: PMC9469649
- DOI: 10.1016/j.bmcl.2022.128841
Development of an automated screen for Kv7.2 potassium channels and discovery of a new agonist chemotype
Abstract
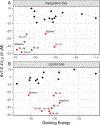
To identify pore domain ligands on Kv7.2 potassium ion channels, we compared wild-type (WT) and W236L mutant Kv7.2 channels in a series of assays with previously validated and novel agonist chemotypes. Positive controls were retigabine, flupirtine, and RL-81; i.e. Kv7.2 channel activators that significantly shift voltage-dependent activation to more negative potentials (ΔV50) at 5 µM. We identified 6 new compounds that exhibited differential enhancing activity between WT and W236L mutant channels. Whole cell patch-clamp electrophysiology studies were conducted to identify Kv7.2. Kv7.2/3, Kv7.4, and Kv7.5 selectivity. Our results validate the SyncroPatch platform and establish new structure activity relationships (SAR). Specifically, in addition to selective Kv7.2, Kv7.2/3, Kv7.4. and Kv7.5 agonists, we identified a novel chemotype, ZK-21, a 4-aminotetrahydroquinoline that is distinct from any of the previously described Kv7 channel modifiers. Using flexible receptor docking, ZK-21 was predicted to be stabilized by W236 and bind perpendicular to retigabine, burying the benzyl carbamate group into a tunnel reaching the core of the pore domain.
Keywords: Agonists; Potassium channels; Quinolines; SyncroPatch screen; Voltage-dependent activation.
Copyright © 2022. Published by Elsevier Ltd.
Figures






References
-
- Jentsch TJ Neuronal KCNQ potassium channels: Physiology and role in disease. Nat. Rev. Neurosci. 2000, 1, 21–30. - PubMed
-
- Zhang Y-M; Xu H-Y; Hu H-N; Tian F-Y; Chen F; Liu H-N; Zhan L; Pi X-P; Liu J; Gao Z-B; Nan F-J Discovery of HN37 as a potent and chemically stable antiepileptic drug candidate. J. Med. Chem. 2021, 64, 5816–5837. - PubMed
-
- Ostacolo C; Miceli F; Di Sarno V; Nappi P; Iraci N; Soldovieri MV; Ciaglia T; Ambrosino P; Vestuto V; Lauritano A; Musella S; Pepe G; Basilicata MG; Manfra M; Perinelli DR; Novellino E; Bertamino A; Gomez-Monterrey IM; Campiglia P; Taglialatela M Synthesis and pharmacological characterization of conformationally restricted retigabine analogues as novel neuronal Kv7 channel activators. J. Med. Chem. 2020, 63, 163–185. - PubMed
-
- Grupe M; Bentzen BH; Benned-Jensen T; Nielsen V; Frederiksen K; Jensen HS; Jacobsen A-M; Skibsbye L; Sams AG; Grunnet M; Rottländer M; Bastlund JF “In vitro and in vivo characterization of Lu AA41178: A novel, brain penetrant, pan-selective Kv7 potassium channel opener with efficacy in preclinical models of epileptic seizures and psychiatric disorders. Eur. J. Pharmacol. 2020, 887, 173440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

