Immunological Interaction of HLA-DPB1 and Proteinase 3 in ANCA Vasculitis is Associated with Clinical Disease Activity
- PMID: 35672132
- PMCID: PMC9342628
- DOI: 10.1681/ASN.2021081142
Immunological Interaction of HLA-DPB1 and Proteinase 3 in ANCA Vasculitis is Associated with Clinical Disease Activity
Abstract
Background: PR3-ANCA vasculitis has a genetic association with HLA-DPB1. We explored immunologic and clinical features related to the interaction of HLA-DPB1*04:01 with a strongly binding PR3 peptide epitope (PR3225-239).
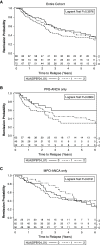
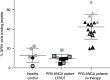
Methods: Patients with ANCA vasculitis with active disease and disease in remission were followed longitudinally. Peripheral blood mononuclear cells from patients and healthy controls with HLA-DPB1*04:01 were tested for HLA-DPB1*04:01 expression and interaction with a PR3 peptide identified via in silico and in vitro assays. Tetramers (HLA/peptide multimers) identified autoreactive T cells in vitro. RESULTS: The HLA-DPB1*04:01 genotype was associated with risk of relapse in PR3-ANCA (HR for relapse 2.06; 95% CI, 1.01 to 4.20) but not in myeloperoxidase (MPO)-ANCA or the combined cohort. In silico predictions of HLA and PR3 peptide interactions demonstrated strong affinity between ATRLFPDFFTRVALY (PR3225-239) and HLA-DPB1*04:01 that was confirmed by in vitro competitive binding studies. The interaction was tested in ex vivo flow cytometry studies of labeled peptide and HLA-DPB1*04:01-expressing cells. We demonstrated PR3225-239 specific autoreactive T cells using synthetic HLA multimers (tetramers). Patients in long-term remission off therapy had autoantigenic peptide and HLA interaction comparable to that of healthy volunteers.
Conclusions: The risk allele HLA-DPB1*04:01 has been associated with PR3-ANCA, but its immunopathologic role was unclear. These studies demonstrate that HLA-DPB1*04:01 and PR3225-239 initiate an immune response. Autoreactive T cells specifically recognized PR3225-239 presented by HLA-DPB1*04:01. Although larger studies should validate these findings, the pathobiology may explain the observed increased risk of relapse in our cohort. Moreover, lack of HLA and autoantigen interaction observed during long-term remission signals immunologic nonresponsiveness.
Keywords: ANCA; HLA; HLA-DPB1 antigen; PR3; T cell; maintenance; remission.
Copyright © 2022 by the American Society of Nephrology.
Figures



Comment in
-
Identifying Antigen-Specific T Cells in ANCA-Associated Vasculitis: A Glimpse of the Future?J Am Soc Nephrol. 2022 Aug;33(8):1435-1437. doi: 10.1681/ASN.2022060668. J Am Soc Nephrol. 2022. PMID: 35906086 Free PMC article. No abstract available.
References
-
- Falk RJ, Jennette JC: ANCA are pathogenic--oh yes they are! J Am Soc Nephrol 13: 1977–1979, 2002 - PubMed
-
- Hagen EC, Ballieux BE, van Es LA, Daha MR, van der Woude FJ: Antineutrophil cytoplasmic autoantibodies: A review of the antigens involved, the assays, and the clinical and possible pathogenetic consequences. Blood 81: 1996–2002, 1993 - PubMed
-
- Free ME, Bunch DO, McGregor JA, Jones BE, Berg EA, Hogan SL, et al. : Patients with antineutrophil cytoplasmic antibody-associated vasculitis have defective Treg cell function exacerbated by the presence of a suppression-resistant effector cell population. Arthritis Rheum 65: 1922–1933, 2013 - PMC - PubMed
-
- Lamprecht P, Wieczorek S, Epplen JT, Ambrosch P, Kallenberg CG: Granuloma formation in ANCA-associated vasculitides. APMIS Suppl 117: 32–36, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

