Microglial mTOR Activation Upregulates Trem2 and Enhances β-Amyloid Plaque Clearance in the 5XFAD Alzheimer's Disease Model
- PMID: 35672148
- PMCID: PMC9270922
- DOI: 10.1523/JNEUROSCI.2427-21.2022
Microglial mTOR Activation Upregulates Trem2 and Enhances β-Amyloid Plaque Clearance in the 5XFAD Alzheimer's Disease Model
Abstract
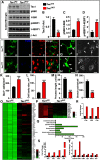
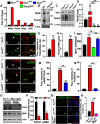
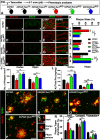
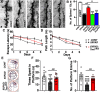
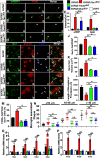
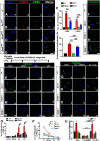
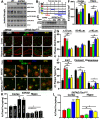
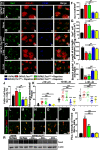
The mechanistic target of rapamycin (mTOR) signaling pathway plays a major role in key cellular processes including metabolism and differentiation; however, the role of mTOR in microglia and its importance in Alzheimer's disease (AD) have remained largely uncharacterized. We report that selective loss of Tsc1, a negative regulator of mTOR, in microglia in mice of both sexes, caused mTOR activation and upregulation of Trem2 with enhanced β-Amyloid (Aβ) clearance, reduced spine loss, and improved cognitive function in the 5XFAD AD mouse model. Combined loss of Tsc1 and Trem2 in microglia led to reduced Aβ clearance and increased Aβ plaque burden revealing that Trem2 functions downstream of mTOR. Tsc1 mutant microglia showed increased phagocytosis with upregulation of CD68 and Lamp1 lysosomal proteins. In vitro studies using Tsc1-deficient microglia revealed enhanced endocytosis of the lysosomal tracker indicator Green DND-26 suggesting increased lysosomal activity. Incubation of Tsc1-deficient microglia with fluorescent-labeled Aβ revealed enhanced Aβ uptake and clearance, which was blunted by rapamycin, an mTOR inhibitor. In vivo treatment of mice of relevant genotypes in the 5XFAD background with rapamycin, affected microglial activity, decreased Trem2 expression and reduced Aβ clearance causing an increase in Aβ plaque burden. Prolonged treatment with rapamycin caused even further reduction of mTOR activity, reduction in Trem2 expression, and increase in Aβ levels. Together, our findings reveal that mTOR signaling in microglia is critically linked to Trem2 regulation and lysosomal biogenesis, and that the upregulation of Trem2 in microglia through mTOR activation could be exploited toward better therapeutic avenues to Aβ-related AD pathologies.SIGNIFICANCE STATEMENT Mechanistic target of rapamycin (mTOR) signaling pathway is a key regulator for major cellular metabolic processes. However, the link between mTOR signaling and Alzheimer's disease (AD) is not well understood. In this study, we provide compelling in vivo evidence that mTOR activation in microglia would benefit β-Amyloid (Aβ)-related AD pathologies, as it upregulates Trem2, a key receptor for Aβ plaque uptake. Inhibition of mTOR pathway with rapamycin, a well-established immunosuppressant, downregulated Trem2 in microglia and reduced Aβ plaque clearance indicating that mTOR inactivation may be detrimental in Aβ-associated AD patients. This finding will have a significant public health impact and benefit, regarding the usage of rapamycin in AD patients, which we believe will aggravate the Aβ-related AD pathologies.
Keywords: Alzheimer's disease; Trem2; mTOR; microglia; rapamycin; β-amyloid.
Copyright © 2022 the authors.
Figures
Similar articles
-
Intermittent hypoxia training enhances Aβ endocytosis by plaque associated microglia via VPS35-dependent TREM2 recycling in murine Alzheimer's disease.Alzheimers Res Ther. 2024 Jun 3;16(1):121. doi: 10.1186/s13195-024-01489-6. Alzheimers Res Ther. 2024. PMID: 38831312 Free PMC article.
-
Facilitating microglial phagocytosis by which Jiawei Xionggui Decoction alleviates cognitive impairment via TREM2-mediated energy metabolic reprogramming.Chin J Nat Med. 2025 Aug;23(8):909-919. doi: 10.1016/S1875-5364(25)60927-7. Chin J Nat Med. 2025. PMID: 40754372
-
Effects of microglial depletion and TREM2 deficiency on Aβ plaque burden and neuritic plaque tau pathology in 5XFAD mice.Acta Neuropathol Commun. 2021 Sep 9;9(1):150. doi: 10.1186/s40478-021-01251-1. Acta Neuropathol Commun. 2021. PMID: 34503586 Free PMC article.
-
Untangling the Role of TREM2 in Conjugation with Microglia in Neuronal Dysfunction: A Hypothesis on a Novel Pathway in the Pathophysiology of Alzheimer's Disease.J Alzheimers Dis. 2023;94(s1):S319-S333. doi: 10.3233/JAD-221070. J Alzheimers Dis. 2023. PMID: 36683512 Free PMC article. Review.
-
New insights into the role of TREM2 in Alzheimer's disease.Mol Neurodegener. 2018 Dec 20;13(1):66. doi: 10.1186/s13024-018-0298-9. Mol Neurodegener. 2018. PMID: 30572908 Free PMC article. Review.
Cited by
-
m5C RNA methylation: a potential mechanism for infectious Alzheimer's disease.Front Cell Dev Biol. 2024 Aug 8;12:1440143. doi: 10.3389/fcell.2024.1440143. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39175875 Free PMC article. Review.
-
The role of SIRT2 inhibition on the aging process of brain in male rats.Aging Brain. 2023 Jul 17;4:100087. doi: 10.1016/j.nbas.2023.100087. eCollection 2023. Aging Brain. 2023. PMID: 37519449 Free PMC article.
-
Cellular senescence in Alzheimer's disease: from physiology to pathology.Transl Neurodegener. 2024 Nov 20;13(1):55. doi: 10.1186/s40035-024-00447-4. Transl Neurodegener. 2024. PMID: 39568081 Free PMC article. Review.
-
Microglia either promote or restrain TRAIL-mediated excitotoxicity caused by Aβ1-42 oligomers.J Neuroinflammation. 2024 Sep 1;21(1):215. doi: 10.1186/s12974-024-03208-2. J Neuroinflammation. 2024. PMID: 39218898 Free PMC article.
-
mTOR Signaling in Macrophages: All Depends on the Context.Int J Mol Sci. 2025 Aug 6;26(15):7598. doi: 10.3390/ijms26157598. Int J Mol Sci. 2025. PMID: 40806725 Free PMC article. Review.
References
-
- Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, Sierra A, Molnár Z, Cragg MS, Garaschuk O, Perry VH, Gomez-Nicola D (2017) Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep 18:391–405. 10.1016/j.celrep.2016.12.041 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
