Camel milk protein hydrosylate alleviates hepatic steatosis and hypertension in high fructose-fed rats
- PMID: 35672152
- PMCID: PMC9176680
- DOI: 10.1080/13880209.2022.2079678
Camel milk protein hydrosylate alleviates hepatic steatosis and hypertension in high fructose-fed rats
Abstract
Context: Camel milk is used in traditional medicine to treat diabetes mellitus hypertension and other metabolic disorders.
Objective: This study evaluated the antisteatotic and antihypertensive effects of camel milk protein hydrolysate (CMH) in high fructose (HF)-fed rats and compared it with the effects afforded by the intact camel milk protein extract (ICM).
Materials and methods: Adult male Wistar rats were divided into 6 groups (n = 8 each) as 1) control, 2) ICM (1000 mg/kg), 3) CMH (1000 mg/kg), 4) HF (15% in drinking water), 5) HF (15%) + ICM (1000 mg/kg), and 6) HF (15%) + CMH (1000 mg/kg). All treatments were given orally for 21 weeks, daily.
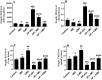
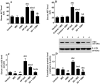
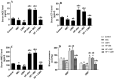
Results: Both ICM and CMH reduced fasting glucose and insulin levels, serum and hepatic levels of cholesterol and triglycerides, and serum levels of ALT and AST, angiotensin II, ACE, endothelin-1, and uric acid in HF-fed rats. In addition, both ICM and CMH reduced hepatic fat deposition in the hepatocytes and reduced hepatocyte damage. This was associated with an increase in the hepatic activity of AMPK, higher PPARα mRNA, reduced expression of fructokinase C, SREBP1, SREBP2, fatty acid synthase, and HMG-CoA-reductase. Both treatments lowered systolic and diastolic blood pressure. However, the effects of CMH on all these parameters were greater as compared to ICM.
Discussion and conclusions: The findings of this study encourage the use of CMH in a large-scale population and clinical studies to treat metabolic steatosis and hypertension.
Keywords: Metabolic disorder; NAFLD; angiotensin; blood pressure; hyperglycaemia; hyperlipidaemia; liver.
Conflict of interest statement
The authors declare no conflicts of interest associated with this work.
Figures



References
-
- Alhaj OA. 2020. Exploring potential therapeutic properties of camel milk. In Alhaj O., Faye B., and Agrawal R. (Eds.), Handbook of research on health and environmental benefits of camel products. Hershey (PA): IGI Global. p. 123–154.
-
- Al-Numair KS. 2010. Type II diabetic rats and the hypolipidemic effect of camel milk. J Food Agric Environ. 8:77–81.
-
- Al-Shamsi KA, Mudgil P, Hassan HM, Maqsood S.. 2018. Camel milk protein hydrolysates with improved technofunctional properties and enhanced antioxidant potential in in vitro and in food model systems. J Dairy Sci. 101(1):47–60. - PubMed
-
- Ayoub MA, Palakkott AR, Ashraf A, Iratni R.. 2018. The molecular basis of the anti-diabetic properties of camel milk. Diabetes Res Clin Pract. 146:305–312. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
