Ginkgo biloba in the management of the COVID-19 severity
- PMID: 35672257
- PMCID: PMC9348126
- DOI: 10.1002/ardp.202200188
Ginkgo biloba in the management of the COVID-19 severity
Abstract
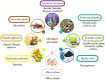
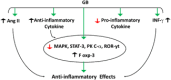
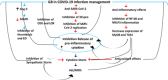
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is linked with inflammatory disorders and the development of oxidative stress in extreme cases. Therefore, anti-inflammatory and antioxidant drugs may alleviate these complications. Ginkgo biloba L. folium extract (EGb) is a herbal medicine containing various active constituents. This review aims to provide a critical discussion on the potential role of EGb in the management of coronavirus disease 2019 (COVID-19). The antiviral effect of EGb is mediated by different mechanisms, including blocking SARS-CoV-2 3-chymotrypsin-like protease that provides trans-variant effectiveness. Moreover, EGb impedes the development of pulmonary inflammatory disorders through the diminution of neutrophil elastase activity, the release of proinflammatory cytokines, platelet aggregation, and thrombosis. Thus, EGb can attenuate the acute lung injury and acute respiratory distress syndrome in COVID-19. In conclusion, EGb offers the potential of being used as adjuvant antiviral and symptomatic therapy. Nanosystems enabling targeted delivery, personalization, and booster of effects provide the opportunity for the use of EGb in modern phytotherapy.
Keywords: Ginkgo biloba; SARS-CoV-2; complementary medicine; nanomedicines; viral proteases.
© 2022 Deutsche Pharmazeutische Gesellschaft.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Discovery of naturally occurring inhibitors against SARS-CoV-2 3CLpro from Ginkgo biloba leaves via large-scale screening.Fitoterapia. 2021 Jul;152:104909. doi: 10.1016/j.fitote.2021.104909. Epub 2021 Apr 22. Fitoterapia. 2021. PMID: 33894315 Free PMC article.
-
EGb 761: ginkgo biloba extract, Ginkor.Drugs R D. 2003;4(3):188-93. doi: 10.2165/00126839-200304030-00009. Drugs R D. 2003. PMID: 12757407 Review.
-
Evidence that Ginkgo Biloba could use in the influenza and coronavirus COVID-19 infections.J Basic Clin Physiol Pharmacol. 2021 Feb 16;32(3):131-143. doi: 10.1515/jbcpp-2020-0310. J Basic Clin Physiol Pharmacol. 2021. PMID: 33594843 Review.
-
Ginkgo biloba--an appraisal.Kathmandu Univ Med J (KUMJ). 2004 Jul-Sep;2(3):225-9. Kathmandu Univ Med J (KUMJ). 2004. PMID: 16400219 Review.
-
Comparative anticancer and antioxidant activities of different ingredients of Ginkgo biloba extract (EGb 761).Planta Med. 2009 Jun;75(8):792-6. doi: 10.1055/s-0029-1185451. Epub 2009 Mar 13. Planta Med. 2009. PMID: 19288403
Cited by
-
Treating COVID-19 with Medicinal Plants: Is It Even Conceivable? A Comprehensive Review.Viruses. 2024 Feb 20;16(3):320. doi: 10.3390/v16030320. Viruses. 2024. PMID: 38543686 Free PMC article. Review.
-
Mixed storm in SARS-CoV-2 infection: A narrative review and new term in the Covid-19 era.Immun Inflamm Dis. 2023 Apr;11(4):e838. doi: 10.1002/iid3.838. Immun Inflamm Dis. 2023. PMID: 37102645 Free PMC article. Review.
-
Insights on Covid-19 with superimposed pulmonary histoplasmosis: The possible nexus.Immun Inflamm Dis. 2023 Sep;11(9):e989. doi: 10.1002/iid3.989. Immun Inflamm Dis. 2023. PMID: 37773721 Free PMC article. Review.
-
Exploring Bioactive Phytomedicines for Advancing Pulmonary Infection Management: Insights and Future Prospects.Phytother Res. 2024 Dec;38(12):5840-5872. doi: 10.1002/ptr.8334. Epub 2024 Oct 9. Phytother Res. 2024. PMID: 39385504 Free PMC article. Review.
-
Ginkgo biloba: Antioxidant Activity and In Silico Central Nervous System Potential.Curr Issues Mol Biol. 2023 Dec 1;45(12):9674-9691. doi: 10.3390/cimb45120604. Curr Issues Mol Biol. 2023. PMID: 38132450 Free PMC article.
References
-
- @JohnsHopkins .COVID‐19 Map—Johns Hopkins Coronavirus Resource Center. 2022. Accessed May 4, 2022. https://coronavirus.jhu.edu/map.html
-
- Yamamoto V., Bolanos J. F., Fiallos J., Strand S. E., Morris K., Shahrokhinia S., Cushing T. R., Hopp L., Tiwari A., Hariri R., Sokolov R., Wheeler C., Kaushik A., Elsayegh A., Eliashiv D., Hedrick R., Jafari B., Johnson J. P., Khorsandi M., Gonzalez N., Balakhani G., Lahiri S., Ghavidel K., Amaya M., Kloor H., Hussain N., Huang E., Cormier J., Wesson Ashford J., Wang J. C., Yaghobian S., Khorrami P., Shamloo B., Moon C., Shadi P., Kateb B., J. Alzheimer's Dis. 2020, 77, 459. 10.3233/JAD-200831 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

