Nisin probiotic prevents inflammatory bone loss while promoting reparative proliferation and a healthy microbiome
- PMID: 35672331
- PMCID: PMC9174264
- DOI: 10.1038/s41522-022-00307-x
Nisin probiotic prevents inflammatory bone loss while promoting reparative proliferation and a healthy microbiome
Abstract
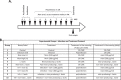
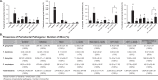
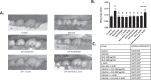
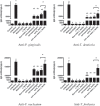
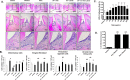
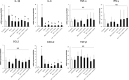
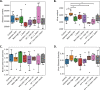
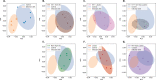
Dysbiosis of the oral microbiome mediates chronic periodontal disease. Realignment of microbial dysbiosis towards health may prevent disease. Treatment with antibiotics and probiotics can modulate the microbial, immunological, and clinical landscape of periodontal disease with some success. Antibacterial peptides or bacteriocins, such as nisin, and a nisin-producing probiotic, Lactococcus lactis, have not been examined in this context, yet warrant examination because of their biomedical benefits in eradicating biofilms and pathogenic bacteria, modulating immune mechanisms, and their safety profile in humans. This study's goal was to examine the potential for nisin and a nisin-producing probiotic to abrogate periodontal bone loss, the host inflammatory response, and changes in oral microbiome composition in a polymicrobial mouse model of periodontal disease. Nisin and a nisin-producing Lactococcus lactis probiotic significantly decreased the levels of several periodontal pathogens, alveolar bone loss, and the oral and systemic inflammatory host response. Surprisingly, nisin and/or the nisin-producing L. lactis probiotic enhanced the population of fibroblasts and osteoblasts despite the polymicrobial infection. Nisin mediated human periodontal ligament cell proliferation dose-dependently by increasing the proliferation marker, Ki-67. Nisin and probiotic treatment significantly shifted the oral microbiome towards the healthy control state; health was associated with Proteobacteria, whereas 3 retroviruses were associated with disease. Disease-associated microbial species were correlated with IL-6 levels. Nisin or nisin-producing probiotic's ability to shift the oral microbiome towards health, mitigate periodontal destruction and the host immune response, and promote a novel proliferative phenotype in reparative connective tissue cells, addresses key aspects of the pathogenesis of periodontal disease and reveals a new biomedical application for nisin in treatment of periodontitis and reparative medicine.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests. The authors M.R.S. and M.J.Z. have an affiliation with Oralome, Inc.
Figures
Similar articles
-
Nisin a probiotic bacteriocin mitigates brain microbiome dysbiosis and Alzheimer's disease-like neuroinflammation triggered by periodontal disease.J Neuroinflammation. 2023 Oct 6;20(1):228. doi: 10.1186/s12974-023-02915-6. J Neuroinflammation. 2023. PMID: 37803465 Free PMC article.
-
Nisin lantibiotic prevents NAFLD liver steatosis and mitochondrial oxidative stress following periodontal disease by abrogating oral, gut and liver dysbiosis.NPJ Biofilms Microbiomes. 2024 Jan 17;10(1):3. doi: 10.1038/s41522-024-00476-x. NPJ Biofilms Microbiomes. 2024. PMID: 38233485 Free PMC article.
-
Nisin and Nisin Probiotic Disrupt Oral Pathogenic Biofilms and Restore Their Microbiome Composition towards Healthy Control Levels in a Peri-Implantitis Setting.Microorganisms. 2022 Jul 1;10(7):1336. doi: 10.3390/microorganisms10071336. Microorganisms. 2022. PMID: 35889055 Free PMC article.
-
Probiotics, including nisin-based probiotics, improve clinical and microbial outcomes relevant to oral and systemic diseases.Periodontol 2000. 2020 Feb;82(1):173-185. doi: 10.1111/prd.12324. Periodontol 2000. 2020. PMID: 31850634 Review.
-
Use of the Probiotic Bifidobacterium animalis subsp. lactis HN019 in Oral Diseases.Int J Mol Sci. 2022 Aug 19;23(16):9334. doi: 10.3390/ijms23169334. Int J Mol Sci. 2022. PMID: 36012597 Free PMC article. Review.
Cited by
-
Effects of sodium lauryl sulfate and postbiotic toothpaste on oral microecology.J Oral Microbiol. 2024 Jun 26;16(1):2372224. doi: 10.1080/20002297.2024.2372224. eCollection 2024. J Oral Microbiol. 2024. PMID: 38939048 Free PMC article.
-
Periodontal treatment and microbiome-targeted therapy in management of periodontitis-related nonalcoholic fatty liver disease with oral and gut dysbiosis.World J Gastroenterol. 2023 Feb 14;29(6):967-996. doi: 10.3748/wjg.v29.i6.967. World J Gastroenterol. 2023. PMID: 36844143 Free PMC article. Review.
-
Periodontal disease and chronic kidney disease: mechanistic insights and novel therapeutic perspectives.Front Cell Infect Microbiol. 2025 Jun 25;15:1611097. doi: 10.3389/fcimb.2025.1611097. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40636262 Free PMC article. Review.
-
Nisin a probiotic bacteriocin mitigates brain microbiome dysbiosis and Alzheimer's disease-like neuroinflammation triggered by periodontal disease.J Neuroinflammation. 2023 Oct 6;20(1):228. doi: 10.1186/s12974-023-02915-6. J Neuroinflammation. 2023. PMID: 37803465 Free PMC article.
-
Regulation of tryptophan-indole metabolic pathway in Porphyromonas gingivalis virulence and microbiota dysbiosis in periodontitis.NPJ Biofilms Microbiomes. 2025 Feb 27;11(1):37. doi: 10.1038/s41522-025-00669-y. NPJ Biofilms Microbiomes. 2025. PMID: 40011497 Free PMC article.
References
-
- WHO. Epidemiology, etiology, and prevention of periodontal diseases. Report of a WHO Scientific Group. World. Health. Organ. Tech. Rep. Ser. 621, 1–60 (1978). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

