Scrutinizing pathogenicity of the USH2A c.2276 G > T; p.(Cys759Phe) variant
- PMID: 35672333
- PMCID: PMC9174243
- DOI: 10.1038/s41525-022-00306-z
Scrutinizing pathogenicity of the USH2A c.2276 G > T; p.(Cys759Phe) variant
Abstract
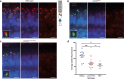
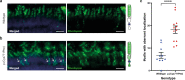
The USH2A variant c.2276 G > T (p.(Cys759Phe)) has been described by many authors as a frequent cause of autosomal recessive retinitis pigmentosa (arRP). However, this is in contrast with the description of two asymptomatic individuals homozygous for this variant. We therefore assessed pathogenicity of the USH2A c.2276 G > T variant using extensive genetic and functional analyses. Whole genome sequencing and optical genome mapping were performed for three arRP cases homozygous for USH2A c.2276 G > T to exclude alternative genetic causes. A minigene splice assay was designed to investigate the effect of c.2276 G > T on pre-mRNA splicing, in presence or absence of the nearby c.2256 T > C variant. Moreover, an ush2ap.(Cys771Phe) zebrafish knock-in model mimicking human p.(Cys759Phe) was generated and characterized using functional and immunohistochemical analyses. Besides the homozygous c.2276 G > T USH2A variant, no alternative genetic causes were identified. Evaluation of the ush2ap.(Cys771Phe) zebrafish model revealed strongly reduced levels of usherin expression at the photoreceptor periciliary membrane, increased levels of rhodopsin localization in the photoreceptor cell body and decreased electroretinogram (ERG) b-wave amplitudes compared to wildtype controls. In conclusion, we confirmed pathogenicity of USH2A c.2276 G > T (p.(Cys759Phe)). Consequently, cases homozygous for c.2276 G > T can now receive a definite genetic diagnosis and can be considered eligible for receiving future QR-421a-mediated exon 13 skipping therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

