Temporal changes in gastrointestinal fungi and the risk of autoimmunity during early childhood: the TEDDY study
- PMID: 35672407
- PMCID: PMC9174155
- DOI: 10.1038/s41467-022-30686-w
Temporal changes in gastrointestinal fungi and the risk of autoimmunity during early childhood: the TEDDY study
Abstract
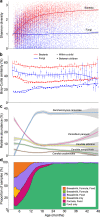
Fungal infections are a major health problem that often begin in the gastrointestinal tract. Gut microbe interactions in early childhood are critical for proper immune responses, yet there is little known about the development of the fungal population from infancy into childhood. Here, as part of the TEDDY (The Environmental Determinants of Diabetes in the Young) study, we examine stool samples of 888 children from 3 to 48 months and find considerable differences between fungi and bacteria. The metagenomic relative abundance of fungi was extremely low but increased while weaning from milk and formula. Overall fungal diversity remained constant over time, in contrast with the increase in bacterial diversity. Fungal profiles had high temporal variation, but there was less variation from month-to-month in an individual than among different children of the same age. Fungal composition varied with geography, diet, and the use of probiotics. Multiple Candida spp. were at higher relative abundance in children than adults, while Malassezia and certain food-associated fungi were lower in children. There were only subtle fungal differences associated with the subset of children that developed islet autoimmunity or type 1 diabetes. Having proper fungal exposures may be crucial for children to establish appropriate responses to fungi and limit the risk of infection: the data here suggests those gastrointestinal exposures are limited and variable.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
- U01 DK063821/DK/NIDDK NIH HHS/United States
- UC4 DK063863/DK/NIDDK NIH HHS/United States
- UL1 TR002535/TR/NCATS NIH HHS/United States
- HHSN267200700014C/DK/NIDDK NIH HHS/United States
- U01 DK063790/DK/NIDDK NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- U01 DK063836/DK/NIDDK NIH HHS/United States
- U01 DK063829/DK/NIDDK NIH HHS/United States
- U01 DK063865/DK/NIDDK NIH HHS/United States
- UC4 DK095300/DK/NIDDK NIH HHS/United States
- UC4 DK063861/DK/NIDDK NIH HHS/United States
- UC4 DK063829/DK/NIDDK NIH HHS/United States
- UC4 DK063821/DK/NIDDK NIH HHS/United States
- UC4 DK117483/DK/NIDDK NIH HHS/United States
- UC4 DK063836/DK/NIDDK NIH HHS/United States
- UC4 DK112243/DK/NIDDK NIH HHS/United States
- U01 DK124166/DK/NIDDK NIH HHS/United States
- U01 DK063861/DK/NIDDK NIH HHS/United States
- P30 ES030285/ES/NIEHS NIH HHS/United States
- U01 DK128847/DK/NIDDK NIH HHS/United States
- UC4 DK063865/DK/NIDDK NIH HHS/United States
- U01 DK063863/DK/NIDDK NIH HHS/United States
- UC4 DK106955/DK/NIDDK NIH HHS/United States
- UC4 DK100238/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

