Platelets from patients with visceral obesity promote colon cancer growth
- PMID: 35672444
- PMCID: PMC9174292
- DOI: 10.1038/s42003-022-03486-7
Platelets from patients with visceral obesity promote colon cancer growth
Abstract
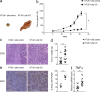
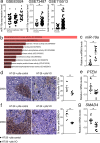
Several studies highlighted the importance of platelets in the tumor microenvironment due to their ability to interact with other cell types such as leukocytes, endothelial, stromal and cancer cells. Platelets can influence tumor development and metastasis formation through several processes consisting of the secretion of growth factors and cytokines and/or via direct interaction with cancer cells and endothelium. Patients with visceral obesity (VO) are susceptible to pro-thrombotic and pro-inflammatory states and to development of cancer, especially colon cancer. These findings provide us with the impetus to analyze the role of platelets isolated from VO patients in tumor growth and progression with the aim to explore a possible link between platelet activation, obesity and colon cancer. Here, using xenograft colon cancer models, we prove that platelets from patients with visceral obesity are able to strongly promote colon cancer growth. Then, sequencing platelet miRNome, we identify miR-19a as the highest expressed miRNA in obese subjects and prove that miR-19a is induced in colon cancer. Last, administration of miR-19a per se in the xenograft colon cancer model is able to promote colon cancer growth. We thus elect platelets with their specific miRNA abundance as important factors in the tumor promoting microenvironment of patients with visceral obesity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

