Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation
- PMID: 35672452
- PMCID: PMC9385498
- DOI: 10.1038/s41385-022-00524-9
Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation
Abstract
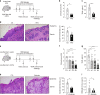
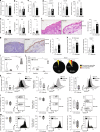
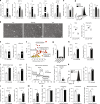
Barrier integrity is central to the maintenance of healthy immunological homeostasis. Impaired skin barrier function is linked with enhanced allergen sensitization and the development of diseases such as atopic dermatitis (AD), which can precede the development of other allergic disorders, for example, food allergies and asthma. Epidemiological evidence indicates that children suffering from allergies have lower levels of dietary fibre-derived short-chain fatty acids (SCFA). Using an experimental model of AD-like skin inflammation, we report that a fermentable fibre-rich diet alleviates systemic allergen sensitization and disease severity. The gut-skin axis underpins this phenomenon through SCFA production, particularly butyrate, which strengthens skin barrier function by altering mitochondrial metabolism of epidermal keratinocytes and the production of key structural components. Our results demonstrate that dietary fibre and SCFA improve epidermal barrier integrity, ultimately limiting early allergen sensitization and disease development.The Graphical Abstract was designed using Servier Medical Art images ( https://smart.servier.com ).
© 2022. The Author(s).
Conflict of interest statement
B.J.M. is part of the editorial board of
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

