Host-directed immunotherapy of viral and bacterial infections: past, present and future
- PMID: 35672482
- PMCID: PMC9171745
- DOI: 10.1038/s41577-022-00734-z
Host-directed immunotherapy of viral and bacterial infections: past, present and future
Abstract
The advent of COVID-19 and the persistent threat of infectious diseases such as tuberculosis, malaria, influenza and HIV/AIDS remind us of the marked impact that infections continue to have on public health. Some of the most effective protective measures are vaccines but these have been difficult to develop for some of these infectious diseases even after decades of research. The development of drugs and immunotherapies acting directly against the pathogen can be equally challenging, and such pathogen-directed therapeutics have the potential disadvantage of selecting for resistance. An alternative approach is provided by host-directed therapies, which interfere with host cellular processes required for pathogen survival or replication, or target the host immune response to infection (immunotherapies) to either augment immunity or ameliorate immunopathology. Here, we provide a historical perspective of host-directed immunotherapeutic interventions for viral and bacterial infections and then focus on SARS-CoV-2 and Mycobacterium tuberculosis, two major human pathogens of the current era, to indicate the key lessons learned and discuss candidate immunotherapeutic approaches, with a focus on drugs currently in clinical trials.
© 2022. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
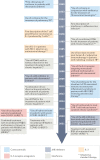
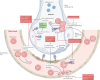
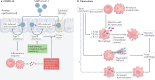

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

