Ashwagandha-loaded nanocapsules improved the behavioral alterations, and blocked MAPK and induced Nrf2 signaling pathways in a hepatic encephalopathy rat model
- PMID: 35672652
- PMCID: PMC9726678
- DOI: 10.1007/s13346-022-01181-y
Ashwagandha-loaded nanocapsules improved the behavioral alterations, and blocked MAPK and induced Nrf2 signaling pathways in a hepatic encephalopathy rat model
Abstract
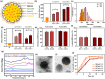
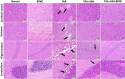
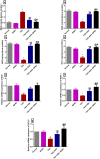
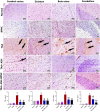
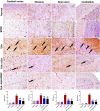
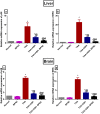
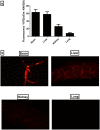
Ashwagandha (ASH), a vital herb in Ayurvedic medicine, demonstrated potent preclinical hepato- and neuroprotective effects. However, its efficacy is limited due to low oral bioavailability. Accordingly, we encapsulated ASH extract in chitosan-alginate bipolymeric nanocapsules (ASH-BPNCs) to enhance its physical stability and therapeutic effectiveness in the gastrointestinal tract. ASH-BPNC was prepared by emulsification followed by sonication. The NCs showed small particle size (< 220 nm), zeta-potential of 25.2 mV, relatively high entrapment efficiency (79%), physical stability at acidic and neutral pH, and in vitro release profile that extended over 48 h. ASH-BPNC was then investigated in a thioacetamide-induced hepatic encephalopathy (HE) rat model. Compared with free ASH, ASH-BPNC improved survival, neurological score, general motor activity, and cognitive task-performance. ASH-BPNC restored ALT, AST and ammonia serum levels, and maintained hepatic and brain architecture. ASH-BPNC also restored GSH, MDA, and glutathione synthetase levels, and Nrf2 and MAPK signaling pathways in liver and brain tissues. Moreover, ASH-BPNC downregulated hepatic NF-κB immunohistochemical expression. Moreover, the in vivo biodistribution studies demonstrated that most of the administered ASH-BPNC is accumulated in the brain and hepatic tissues. In conclusion, chitosan-alginate BPNCs enhanced the hepatoprotective and neuroprotective effects of ASH, thus providing a promising therapeutic approach for HE.
Keywords: Ashwagandha; Bipolymeric nanocapsules; Cognition; Hepatic encephalopathy; MAPK pathway; Nrf2 pathway.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways.J Ethnopharmacol. 2021 Sep 15;277:114141. doi: 10.1016/j.jep.2021.114141. Epub 2021 Apr 24. J Ethnopharmacol. 2021. PMID: 33905819
-
Flavonoids from Barnebydendron riedelii leaf extract mitigate thioacetamide-induced hepatic encephalopathy in rats: The interplay of NF-κB/IL-6 and Nrf2/HO-1 signaling pathways.Bioorg Chem. 2020 Dec;105:104444. doi: 10.1016/j.bioorg.2020.104444. Epub 2020 Nov 1. Bioorg Chem. 2020. PMID: 33197852
-
Protective role of chrysin on thioacetamide-induced hepatic encephalopathy in rats.Chem Biol Interact. 2019 Feb 1;299:111-119. doi: 10.1016/j.cbi.2018.11.021. Epub 2018 Nov 28. Chem Biol Interact. 2019. PMID: 30500344
-
SB332235, a CXCR2 antagonist, ameliorates thioacetamide-induced hepatic encephalopathy through modulation of the PI3K/AKT pathways in rats.Neurotoxicology. 2022 Sep;92:110-121. doi: 10.1016/j.neuro.2022.08.005. Epub 2022 Aug 10. Neurotoxicology. 2022. PMID: 35961375
-
BabaoDan cures hepatic encephalopathy by decreasing ammonia levels and alleviating inflammation in rats.J Ethnopharmacol. 2020 Mar 1;249:112301. doi: 10.1016/j.jep.2019.112301. Epub 2019 Oct 14. J Ethnopharmacol. 2020. PMID: 31622746
Cited by
-
Anthelmintic and Hepatoprotective Activities of the Green-Synthesized Zinc Oxide Nanoparticles Against Parascaris equorum Infection in Rats.Acta Parasitol. 2024 Mar;69(1):283-301. doi: 10.1007/s11686-023-00728-4. Epub 2023 Dec 6. Acta Parasitol. 2024. PMID: 38057445 Free PMC article.
-
Phenolic-rich extracts of Teucrium oliverianum confer protection against thioacetamide-induced liver fibrosis in rats: Insights from metabolomics, biochemical and histopathological analysis.PLoS One. 2025 Sep 2;20(9):e0330595. doi: 10.1371/journal.pone.0330595. eCollection 2025. PLoS One. 2025. PMID: 40892759 Free PMC article.
-
Hypericum perforatum L. Nanoemulsion Mitigates Cisplatin-Induced Chemobrain via Reducing Neurobehavioral Alterations, Oxidative Stress, Neuroinflammation, and Apoptosis in Adult Rats.Toxics. 2023 Feb 8;11(2):159. doi: 10.3390/toxics11020159. Toxics. 2023. PMID: 36851034 Free PMC article.
-
Effects of ashwagandha (Withania somnifera) root extract on aging-related changes in healthy geriatric dogs: A randomized, double-blinded placebo-controlled study.Vet Med Sci. 2024 Sep;10(5):e1556. doi: 10.1002/vms3.1556. Vet Med Sci. 2024. PMID: 39078383 Free PMC article.
-
Anthelmintic and therapeutic effects of the biogenic zinc oxide nanoparticles against acute kidney injury induced by Parascaris equorum Infection in rats.J Parasit Dis. 2024 Mar;48(1):14-24. doi: 10.1007/s12639-023-01637-z. Epub 2023 Nov 24. J Parasit Dis. 2024. PMID: 38440749 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

