A recombinant Bifidobacterium bifidum BGN4 strain expressing the streptococcal superoxide dismutase gene ameliorates inflammatory bowel disease
- PMID: 35672695
- PMCID: PMC9172062
- DOI: 10.1186/s12934-022-01840-2
A recombinant Bifidobacterium bifidum BGN4 strain expressing the streptococcal superoxide dismutase gene ameliorates inflammatory bowel disease
Abstract
Background: Inflammatory bowel disease (IBD) is a gastrointestinal disease characterized by diarrhea, rectal bleeding, abdominal pain, and weight loss. Recombinant probiotics producing specific proteins with IBD therapeutic potential are currently considered novel drug substitutes. In this study, a Bifidobacterium bifidum BGN4-SK strain was designed to produce the antioxidant enzymes streptococcal superoxide dismutase (SOD) and lactobacillus catalase (CAT), and a B. bifidum BGN4-pBESIL10 strain was proposed to generate an anti-inflammatory cytokine, human interleukin (IL)-10. In vitro and in vivo efficacy of these genetically modified Bifidobacterium strains were evaluated for colitis amelioration.
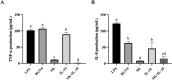
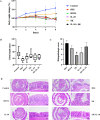
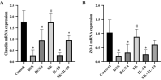
Results: In a lipopolysaccharide (LPS)-stimulated HT-29 cell model, tumor necrosis factor (TNF)-α and IL-8 production was significantly suppressed in the B. bifidum BGN4-SK treatment, followed by B. bifidum BGN4-pBESIL10 treatment, when compared to the LPS-treated control. Synergistic effects on TNF-α suppression were also observed. In a dextran sodium sulphate (DSS)-induced colitis mouse model, B. bifidum BGN4-SK treatment significantly enhanced levels of antioxidant enzymes SOD, glutathione peroxidase (GSH-Px) and CAT, compared to the DSS-only group. B. bifidum BGN4-SK significantly ameliorated the symptoms of DSS-induced colitis, increased the expression of tight junction genes (claudin and ZO-1), and decreased pro-inflammatory cytokines IL-6, IL-1β and TNF-α.
Conclusions: These findings suggest that B. bifidum BGN4-SK ameliorated DSS-induced colitis by generating antioxidant enzymes, maintaining the epithelial barrier, and decreasing the production of pro-inflammatory cytokines. Although B. bifidum BGN4-pBESIL10 exerted anti-inflammatory effects in vitro, the enhancement of IL-10 production and alleviation of colitis were very limited.
Keywords: Bifidobacterium bifidum; Catalase; Human interleukin-10; Inflammatory bowel disease; Superoxide peroxidase.
© 2022. The Author(s).
Conflict of interest statement
Myeong Soo Park is directly employed by BIFIDO Co., Ltd. and he also hold BIFIDO Co., Ltd stocks as a CTO. Other authors declare no conflicts of interest.
Figures

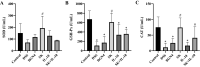
Similar articles
-
Production of biologically active human interleukin-10 by Bifidobacterium bifidum BGN4.Microb Cell Fact. 2021 Jan 19;20(1):16. doi: 10.1186/s12934-020-01505-y. Microb Cell Fact. 2021. PMID: 33468130 Free PMC article.
-
Bifidobacterium bifidum BGN4 Paraprobiotic Supplementation Alleviates Experimental Colitis by Maintaining Gut Barrier and Suppressing Nuclear Factor Kappa B Activation Signaling Molecules.J Med Food. 2022 Feb;25(2):146-157. doi: 10.1089/jmf.2021.K.0150. J Med Food. 2022. PMID: 35148194
-
Antioxidant and Anti-Inflammatory Properties of Recombinant Bifidobacterium bifidum BGN4 Expressing Antioxidant Enzymes.Microorganisms. 2021 Mar 13;9(3):595. doi: 10.3390/microorganisms9030595. Microorganisms. 2021. PMID: 33805797 Free PMC article.
-
Review on Bifidobacterium bifidum BGN4: Functionality and Nutraceutical Applications as a Probiotic Microorganism.Int J Mol Sci. 2016 Sep 14;17(9):1544. doi: 10.3390/ijms17091544. Int J Mol Sci. 2016. PMID: 27649150 Free PMC article. Review.
-
Exploring the Impact of Cyanidin-3-Glucoside on Inflammatory Bowel Diseases: Investigating New Mechanisms for Emerging Interventions.Int J Mol Sci. 2023 May 28;24(11):9399. doi: 10.3390/ijms24119399. Int J Mol Sci. 2023. PMID: 37298350 Free PMC article. Review.
Cited by
-
Antioxidants as Protection against Reactive Oxidative Stress in Inflammatory Bowel Disease.Metabolites. 2023 Apr 18;13(4):573. doi: 10.3390/metabo13040573. Metabolites. 2023. PMID: 37110231 Free PMC article. Review.
-
The Therapeutic Role of Short-Chain Fatty Acids Mediated Very Low-Calorie Ketogenic Diet-Gut Microbiota Relationships in Paediatric Inflammatory Bowel Diseases.Nutrients. 2022 Oct 3;14(19):4113. doi: 10.3390/nu14194113. Nutrients. 2022. PMID: 36235765 Free PMC article. Review.
-
Targeting the Intestinal Microbiota: A Novel Direction in the Treatment of Inflammatory Bowel Disease.Biomedicines. 2024 Oct 15;12(10):2340. doi: 10.3390/biomedicines12102340. Biomedicines. 2024. PMID: 39457652 Free PMC article. Review.
-
Probiotics for the treatment of ulcerative colitis: a review of experimental research from 2018 to 2022.Front Microbiol. 2023 Jul 6;14:1211271. doi: 10.3389/fmicb.2023.1211271. eCollection 2023. Front Microbiol. 2023. PMID: 37485519 Free PMC article. Review.
-
Lactobacillus plantae Expressing Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Single-Chain Antibody Can Inhibit PRRSV Replication and Change the Intestinal Flora Structure of Piglets.Int J Mol Sci. 2025 Mar 3;26(5):2257. doi: 10.3390/ijms26052257. Int J Mol Sci. 2025. PMID: 40076879 Free PMC article.
References
-
- Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–2737. - PubMed
-
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014 doi: 10.1038/nrgastro.2014.66. - DOI - PubMed
-
- Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL# 3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218. doi: 10.1038/ajg.2010.218. - DOI - PMC - PubMed
-
- D’Incà R, Barollo M, Scarpa M, Grillo AR, Brun P, Vettorato MG, Castagliuolo I, Sturniolo GC. Rectal administration of Lactobacillus casei DG modifies flora composition and toll-like receptor expression in colonic mucosa of patients with mild ulcerative colitis. Dig Dis Sci. 2011;56:1178–1187. doi: 10.1007/s10620-010-1384-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- XBTK-2021004/Collaborative Grant-in-Aid of the HBUT National "111" Center for Cellular Regulation and Molecular Pharmaceutics
- XJ2021000101/Doctoral start-up fund of Hubei University of Technology
- S2848321/Regional Specialized Industry Development Program
- S2848321/Regional Specialized Industry Development Program
- No. 221745/Faculty Research and Creative Activity Committee
LinkOut - more resources
Full Text Sources
Miscellaneous

