Synergistic anti-proliferative activity of JQ1 and GSK2801 in triple-negative breast cancer
- PMID: 35672711
- PMCID: PMC9173973
- DOI: 10.1186/s12885-022-09690-2
Synergistic anti-proliferative activity of JQ1 and GSK2801 in triple-negative breast cancer
Abstract
Background: Triple-negative breast cancer (TNBC) constitutes 10-20% of breast cancers and is challenging to treat due to a lack of effective targeted therapies. Previous studies in TNBC cell lines showed in vitro growth inhibition when JQ1 or GSK2801 were administered alone, and enhanced activity when co-administered. Given their respective mechanisms of actions, we hypothesized the combinatorial effect could be due to the target genes affected. Hence the target genes were characterized for their expression in the TNBC cell lines to prove the combinatorial effect of JQ1 and GSK2801.
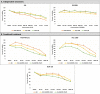
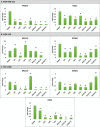
Methods: RNASeq data sets of TNBC cell lines (MDA-MB-231, HCC-1806 and SUM-159) were analyzed to identify the differentially expressed genes in single and combined treatments. The topmost downregulated genes were characterized for their downregulated expression in the TNBC cell lines treated with JQ1 and GSK2801 under different dose concentrations and combinations. The optimal lethal doses were determined by cytotoxicity assays. The inhibitory activity of the drugs was further characterized by molecular modelling studies.
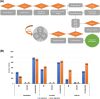
Results: Global expression profiling of TNBC cell lines using RNASeq revealed different expression patterns when JQ1 and GSK2801 were co-administered. Functional enrichment analyses identified several metabolic pathways (i.e., systemic lupus erythematosus, PI3K-Akt, TNF, JAK-STAT, IL-17, MAPK, Rap1 and signaling pathways) enriched with upregulated and downregulated genes when combined JQ1 and GSK2801 treatment was administered. RNASeq identified downregulation of PTPRC, MUC19, RNA5-8S5, KCNB1, RMRP, KISS1 and TAGLN (validated by RT-qPCR) and upregulation of GPR146, SCARA5, HIST2H4A, CDRT4, AQP3, MSH5-SAPCD1, SENP3-EIF4A1, CTAGE4 and RNASEK-C17orf49 when cells received both drugs. In addition to differential gene regulation, molecular modelling predicted binding of JQ1 and GSK2801 with PTPRC, MUC19, KCNB1, TAGLN and KISS1 proteins, adding another mechanism by which JQ1 and GSK2801 could elicit changes in metabolism and proliferation.
Conclusion: JQ1-GSK2801 synergistically inhibits proliferation and results in selective gene regulation. Besides suggesting that combinatorial use could be useful therapeutics for the treatment of TNBC, the findings provide a glimpse into potential mechanisms of action for this combination therapy approach.
Keywords: Breast cancer; Differential expression analysis; Drug resistance; Expression studies; MTT assay; RNASeq.
© 2022. The Author(s).
Conflict of interest statement
The authors have no competing interest to disclose.
Figures





Similar articles
-
Identification of a stemness-related gene panel associated with BET inhibition in triple negative breast cancer.Cell Oncol (Dordr). 2020 Jun;43(3):431-444. doi: 10.1007/s13402-020-00497-6. Epub 2020 Mar 12. Cell Oncol (Dordr). 2020. PMID: 32166583 Free PMC article.
-
GSK2801, a BAZ2/BRD9 Bromodomain Inhibitor, Synergizes with BET Inhibitors to Induce Apoptosis in Triple-Negative Breast Cancer.Mol Cancer Res. 2019 Jul;17(7):1503-1518. doi: 10.1158/1541-7786.MCR-18-1121. Epub 2019 Apr 18. Mol Cancer Res. 2019. PMID: 31000582 Free PMC article.
-
Anti-tumor activity of BET inhibitors in androgen-receptor-expressing triple-negative breast cancer.Sci Rep. 2019 Sep 16;9(1):13305. doi: 10.1038/s41598-019-49366-9. Sci Rep. 2019. PMID: 31527644 Free PMC article.
-
Comprehensive exploration of JQ1 and GSK2801 targets in breast cancer using network pharmacology and molecular modeling approaches.Comput Struct Biotechnol J. 2023 Jun 3;21:3224-3233. doi: 10.1016/j.csbj.2023.06.003. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 38213901 Free PMC article.
-
Single-cell trajectory analysis reveals a CD9 positive state to contribute to exit from stem cell-like and embryonic diapause states and transit to drug-resistant states.Cell Death Discov. 2023 Aug 4;9(1):285. doi: 10.1038/s41420-023-01586-9. Cell Death Discov. 2023. PMID: 37542044 Free PMC article. Review.
Cited by
-
p53 Gain-of-Function Mutation Induces Metastasis via BRD4-Dependent CSF-1 Expression.Cancer Discov. 2023 Dec 12;13(12):2632-2651. doi: 10.1158/2159-8290.CD-23-0601. Cancer Discov. 2023. PMID: 37676642 Free PMC article.
-
Identification of adipose-proximal biomarkers in breast cancer using weighted gene co-expression network analysis.Protoplasma. 2025 Jun 11. doi: 10.1007/s00709-025-02081-x. Online ahead of print. Protoplasma. 2025. PMID: 40498134
-
Combined signature of G protein-coupled receptors and tumor microenvironment provides a prognostic and therapeutic biomarker for skin cutaneous melanoma.J Cancer Res Clin Oncol. 2023 Dec;149(20):18135-18160. doi: 10.1007/s00432-023-05486-4. Epub 2023 Nov 25. J Cancer Res Clin Oncol. 2023. PMID: 38006451 Free PMC article.
-
PTPRC promoted CD8+ T cell mediated tumor immunity and drug sensitivity in breast cancer: based on pan-cancer analysis and artificial intelligence modeling of immunogenic cell death-based drug sensitivity stratification.Front Immunol. 2023 Jun 14;14:1145481. doi: 10.3389/fimmu.2023.1145481. eCollection 2023. Front Immunol. 2023. PMID: 37388747 Free PMC article.
-
GSK2801 Reverses Paclitaxel Resistance in Anaplastic Thyroid Cancer Cell Lines through MYCN Downregulation.Int J Mol Sci. 2023 Mar 22;24(6):5993. doi: 10.3390/ijms24065993. Int J Mol Sci. 2023. PMID: 36983070 Free PMC article.
References
-
- Boyle P, Levin B: World cancer report 2008: IARC Press, International Agency for Research on Cancer; 2008. https://www.cabdirect.org/cabdirect/abstract/20103010665.
-
- Narod SA, Iqbal J, Miller AB. Why have breast cancer mortality rates declined? J Cancer Policy. 2015;5:8–17. doi: 10.1016/j.jcpo.2015.03.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

