PBRM1 deficiency oncogenic addiction is associated with activated AKT-mTOR signalling and aerobic glycolysis in clear cell renal cell carcinoma cells
- PMID: 35672925
- PMCID: PMC9279584
- DOI: 10.1111/jcmm.17418
PBRM1 deficiency oncogenic addiction is associated with activated AKT-mTOR signalling and aerobic glycolysis in clear cell renal cell carcinoma cells
Abstract
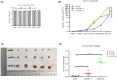
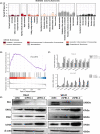
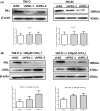
The PBRM1 (PB1) gene which encodes the specific subunit BAF180 of the PBAF SWI/SNF complex, is highly mutated (~ 40%) in clear cell renal cell carcinoma (ccRCC). However, its functions and impact on cell signalling are still not fully understood. Aerobic glycolysis, also known as the 'Warburg Effect', is a hallmark of cancer, whether PB1 is involved in this metabolic shift in clear cell renal cell carcinoma remains unclear. Here, with established stable knockdown PB1 cell lines, we performed functional assays to access the effects on 786-O and SN12C cells. Based on the RNA-seq data, we selected some genes encoding key glycolytic enzymes, including PFKP, ENO1, PKM and LDHA, and examined the expression levels. The AKT-mTOR signalling pathway activity and expression of HIF1α were also analysed. Our data demonstrate that PB1 deficiency promotes the proliferation, migration, Xenograft growth of 786-O and SN12C cells. Notably, knockdown of PB1 activates AKT-mTOR signalling and increases the expression of key glycolytic enzymes at both mRNA and protein levels. Furthermore, we provide evidence that deficient PB1 and hypoxic conditions exert a synergistic effect on HIF 1α expression and lactate production. Thus, our study provides novel insights into the roles of tumour suppressor PB1 and suggests that the AKT-mTOR signalling pathway, as well as glycolysis, is a potential drug target for ccRCC patients with deficient PB1.
Keywords: PBRM1(PB1); AKT-mTOR signalling; HIF1α; aerobic glycolysis; ccRCC.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Yu TA, Hui XI, You‐Fu PA. Research progress on the tumor suppressor gene PBRM1 in renal cell carcinoma. Med J Chin People's Lib Army. 2021;46(7):724‐730.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

