Heteroleptic metallosupramolecular aggregates / complexation for supramolecular catalysis
- PMID: 35673407
- PMCID: PMC9152274
- DOI: 10.3762/bjoc.18.62
Heteroleptic metallosupramolecular aggregates / complexation for supramolecular catalysis
Abstract
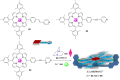
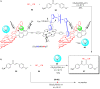
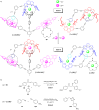
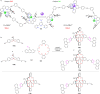
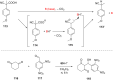
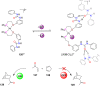
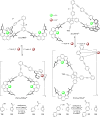
Supramolecular catalysis is reviewed with an eye on heteroleptic aggregates/complexation. Since most of the current metallosupramolecular catalytic systems are homoleptic in nature, the idea of breaking/reducing symmetry has ignited a vivid search for heteroleptic aggregates that are made up by different components. Their higher degree of functional diversity and structural heterogeneity allows, as demonstrated by Nature by the multicomponent ATP synthase motor, a more detailed and refined configuration of purposeful machinery. Furthermore, (metallo)supramolecular catalysis is shown to extend beyond the single "supramolecular unit" and to reach far into the field and concepts of systems chemistry and information science.
Keywords: heteroleptic complexation; information science; supramolecular catalysis; switching catalysis; systems chemistry.
Copyright © 2022, Howlader and Schmittel.
Figures


















Similar articles
-
Multicomponent Self-Assembly of Metallo-Supramolecular Macrocycles and Cages through Dynamic Heteroleptic Terpyridine Complexation.Chemistry. 2018 Jul 2;24(37):9274-9284. doi: 10.1002/chem.201801753. Epub 2018 Jun 13. Chemistry. 2018. PMID: 29714039
-
A Lantern-Shaped Pd(II) Cage Constructed from Four Different Low-Symmetry Ligands with Positional and Orientational Control: An Ancillary Pairings Approach.Angew Chem Int Ed Engl. 2023 Dec 4;62(49):e202314378. doi: 10.1002/anie.202314378. Epub 2023 Oct 31. Angew Chem Int Ed Engl. 2023. PMID: 37816684
-
Self-organization of metallo-supramolecular polymer networks by free formation of pyridine-phenanthroline heteroleptic complexes.Soft Matter. 2023 Nov 1;19(42):8112-8123. doi: 10.1039/d3sm01136d. Soft Matter. 2023. PMID: 37846598
-
Supramolecular chemistry-general principles and selected examples from anion recognition and metallosupramolecular chemistry.Naturwissenschaften. 2007 Dec;94(12):951-66. doi: 10.1007/s00114-007-0282-7. Epub 2007 Jul 24. Naturwissenschaften. 2007. PMID: 17646953 Review.
-
Palladium(II)-Based Self-Assembled Heteroleptic Coordination Architectures: A Growing Family.Chemistry. 2019 Sep 20;25(53):12241-12269. doi: 10.1002/chem.201900831. Epub 2019 Jul 12. Chemistry. 2019. PMID: 31158303 Review.
Cited by
-
Exploring the Gas-Phase Formation and Chemical Reactivity of Highly Reduced M8 L6 Coordination Cages.Angew Chem Int Ed Engl. 2022 Nov 7;61(45):e202212710. doi: 10.1002/anie.202212710. Epub 2022 Oct 7. Angew Chem Int Ed Engl. 2022. PMID: 36102176 Free PMC article.
-
Assembling a new generation of radiopharmaceuticals with supramolecular theranostics.Nat Rev Chem. 2024 Dec;8(12):893-914. doi: 10.1038/s41570-024-00657-4. Epub 2024 Oct 28. Nat Rev Chem. 2024. PMID: 39468298 Review.
-
Orientational self-sorting in cuboctahedral Pd cages.Chem Sci. 2022 Sep 30;13(40):11912-11917. doi: 10.1039/d2sc03856k. eCollection 2022 Oct 19. Chem Sci. 2022. PMID: 36320919 Free PMC article.
-
Supramolecular approaches to mediate chemical reactivity.Beilstein J Org Chem. 2022 Oct 14;18:1463-1465. doi: 10.3762/bjoc.18.152. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 36300008 Free PMC article. No abstract available.
References
Publication types
LinkOut - more resources
Full Text Sources
