N-heterocyclic carbene-stabilized metal nanoparticles within porous organic cages for catalytic application
- PMID: 35673537
- PMCID: PMC9166563
- DOI: 10.1093/nsr/nwac067
N-heterocyclic carbene-stabilized metal nanoparticles within porous organic cages for catalytic application
Abstract
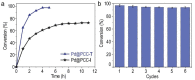
Tuning the surface-embellishing ligands of metal nanoparticles (NPs) is a powerful strategy to modulate their morphology and surface electronic and functional features, impacting their catalytic activity and selectivity. In this work, we report the design and synthesis of a polytriazolium organic cage PIC-T, capable of stabilizing PdNPs within its discrete cavity. The obtained material (denoted Pd@PCC-T) is highly durable and monodispersed with narrow particle-size distribution of 2.06 ± 0.02 nm, exhibiting excellent catalytic performance and recyclability in the Sonogashira coupling and tandem reaction to synthesize benzofuran derivatives. Further investigation indicates that the modulation of N-heterocyclic carbene sites embedded in the organic cage has an impact on NPs' catalytic efficiency, thus providing a novel methodology to design superior NP catalysts.
Keywords: N-heterocyclic carbene; catalytic reaction; metal nanoparticle; porous organic cage.
© The Author(s) 2022. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous
