Exosomal EPHA2 derived from highly metastatic breast cancer cells promotes angiogenesis by activating the AMPK signaling pathway through Ephrin A1-EPHA2 forward signaling
- PMID: 35673569
- PMCID: PMC9169374
- DOI: 10.7150/thno.72404
Exosomal EPHA2 derived from highly metastatic breast cancer cells promotes angiogenesis by activating the AMPK signaling pathway through Ephrin A1-EPHA2 forward signaling
Abstract
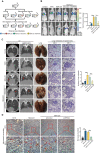
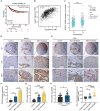
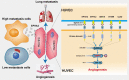
Rationale: Angiogenesis is a fundamental process of tumorigenesis, growth, invasion and metastatic spread. Extracellular vesicles, especially exosomes, released by primary tumors promote angiogenesis and cancer progression. However, the mechanism underlying the pro-angiogenic potency of cancer cell-derived exosomes remains poorly understood. Methods: Exosomes were isolated from breast cancer cells with high metastatic potential (HM) and low metastatic potential (LM). The pro-angiogenic effects of these exosomes were evaluated by in vitro tube formation assays, wound healing assays, rat arterial ring budding assays and in vivo Matrigel plug assays. Subsequently, RNA sequencing, shRNA-mediated gene knockdown, overexpression of different EPHA2 mutants, and small-molecule inhibitors were used to analyze the angiogenesis-promoting effect of exosomal EPHA2 and its potential downstream mechanism. Finally, xenograft tumor models were established using tumor cells expressing different levels of EPHA2 to mimic the secretion of exosomes by tumor cells in vivo, and the metastasis of cancer cells were monitored using the IVIS Spectrum imaging system and Computed Tomography. Results: Herein, we demonstrated that exosomes produced by HM breast cancer cells can promote angiogenesis and metastasis. EPHA2 was rich in HM-derived exosomes and conferred the pro-angiogenic effect. Exosomal EPHA2 can be transferred from HM breast cancer cells to endothelial cells. Moreover, it can stimulate the migration and tube-forming abilities of endothelial cells in vitro and promote angiogenesis and tumor metastasis in vivo. Mechanistically, exosomal EPHA2 activates the AMPK signaling via the ligand Ephrin A1-dependent canonical forward signaling pathway. Moreover, inhibition of the AMPK signaling impairs exosomal EPHA2-mediated pro-angiogenic effects. Conclusion: Our findings identify a novel mechanism of exosomal EPHA2-mediated intercellular communication from breast cancer cells to endothelial cells in the tumor microenvironment to provoke angiogenesis and metastasis. Targeting the exosomal EPHA2-AMPK signaling may serve as a potential strategy for breast cancer therapy.
Keywords: EPHA2; angiogenesis; breast cancer; exosomes; high metastatic potential cells.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Arnedos M, Vicier C, Loi S, Lefebvre C, Michiels S, Bonnefoi H. et al. Precision medicine for metastatic breast cancer-limitations and solutions. Nat Rev Clin Oncol. 2015;12:693–704. - PubMed
-
- Liu MC, Cortés J, O'Shaughnessy J. Challenges in the treatment of hormone receptor-positive, HER2-negative metastatic breast cancer with brain metastases. Cancer Metastasis Rev. 2016;35:323–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

