Current update on theranostic roles of cyclophilin A in kidney diseases
- PMID: 35673572
- PMCID: PMC9169364
- DOI: 10.7150/thno.72948
Current update on theranostic roles of cyclophilin A in kidney diseases
Abstract
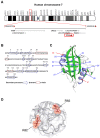
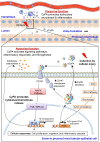
Cyclophilin A (CyPA) or peptidylprolyl isomerase A (PPIA), an immunophilin with peptidyl-prolyl cis/trans isomerase (PPIase) activity, is an abundant cellular protein widely expressed across various cell types and tissues, including the kidney. Expression of CyPA in the kidney is relatively higher in proximal tubular epithelial cells than others along the nephron. Alterations in expression and secretion of CyPA play important roles in physiological processes and pathophysiology of several diseases affecting the kidney. Herein, we provide a brief overview of CyPA structural biology and present the current update on its theranostic roles in various kidney diseases, including diabetic nephropathy, acute kidney injury, chronic kidney disease, renal fibrosis, and nephrotoxicity associated with organ transplantation. Notably, the diagnostic/prognostic role for urinary CyPA in several of these kidney diseases is promising. Finally, future perspectives on the CyPA research, especially targeting CyPA for therapeutics, are discussed. A comprehensive understanding of the theranostic roles of CyPA in kidney diseases is expected to provide novel insights into the design of new therapeutic interventions and prevention strategies.
Keywords: AKI; Biomarker; CKD; Diabetic nephropathy; Nephrotoxicity; PPIA; Renal fibrosis; Therapeutics.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Fischer G, Bang H, Mech C. Determination of enzymatic catalysis for the cis-trans-isomerization of peptide binding in proline-containing peptides. Biomed Biochim Acta. 1984;43:1101–11. - PubMed
-
- Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–7. - PubMed
-
- Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid FX. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989;337:476–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

