Highly elastic 3D-printed gelatin/HA/placental-extract scaffolds for bone tissue engineering
- PMID: 35673575
- PMCID: PMC9169369
- DOI: 10.7150/thno.73146
Highly elastic 3D-printed gelatin/HA/placental-extract scaffolds for bone tissue engineering
Abstract
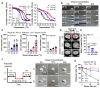
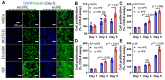
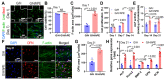
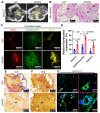
Bioengineering scaffolds have been improved to achieve efficient regeneration of various damaged tissues. In this study, we attempted to fabricate mechanically and biologically activated 3D printed scaffold in which porous gelatin/hydroxyapatite (G/H) as a matrix material provided outstanding mechanical properties with recoverable behavior, and human placental extracts (hPE) embedded in the scaffold were used as bioactive components. Methods: Various cell types (human adipose-derived stem cells; hASCs, pre-osteoblast; MC3T3-E1, human endothelial cell line; EA.hy926, and human dermal fibroblast; hDFs) were used to assess the effect of the hPE on cellular responses. High weight fraction (~ 70 wt%) of hydroxyapatite (HA) in a gelatin solution supplemented with glycerol was used for the G/H scaffold fabrication, and the scaffolds were immersed in hPE for the embedding (G/H/hPE scaffold). The osteogenic abilities of the scaffolds were investigated in cultured cells (hASCs) assaying for ALP activity and expression of osteogenic genes. For the in vivo test, the G/H and G/H/hPE scaffolds were implanted in the rat mastoid obliteration model. Results: The G/H/hPE scaffold presented unique elastic recoverable properties, which are important for efficient usage of implantable scaffolds. The effects of G/H and G/H/hPE scaffold on various in vitro cell-activities including non-toxicity, biocompatibility, and cell proliferation were investigated. The in vitro results indicated that proliferation (G/H = 351.1 ± 13.3%, G/H/hPE = 430.9 ± 8.7% at day 14) and expression of osteogenic markers (ALP: 3.4-fold, Runx2: 3.9-fold, BMP2: 1.7-fold, OPN: 2.4-fold, and OCN: 4.8-fold at day 21) of hASCs grown in the G/H/hPE scaffold were significantly enhanced compared with that in cells grown in the G/H scaffold. In addition, bone formation was also observed in an in vivo model using rat mastoid obliteration. Conclusion:In vitro and in vivo results suggested that the G/H/hPE scaffold is a potential candidate for use in bone tissue engineering.
Keywords: bone; gelatin; placental-extracts; scaffold; tissue engineering.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Griffin KS, Davis KM, McKinley TO, Anglen JO, Chu T-MG, Boerckel JD. et al. Evolution of bone grafting: bone grafts and tissue engineering strategies for vascularized bone regeneration. Clin Rev Bone Miner Metab. 2015;13:232–44.
-
- Baldwin P, Li DJ, Auston DA, Mir HS, Yoon RS, Koval KJ. Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J Orthop Trauma. 2019;33:203–13. - PubMed
-
- Wang Z, Wang Y, Yan J, Zhang K, Lin F, Xiang L. et al. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv Drug Deliv Rev. 2021;174:504–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

