Image-guided drug delivery of nanotheranostics for targeted lung cancer therapy
- PMID: 35673583
- PMCID: PMC9169367
- DOI: 10.7150/thno.72803
Image-guided drug delivery of nanotheranostics for targeted lung cancer therapy
Abstract
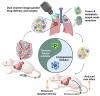
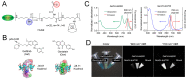
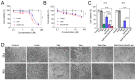
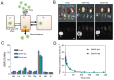
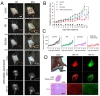
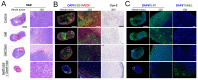
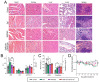
Enormous efforts have been made to integrate various therapeutic interventions into multifunctional nanoplatforms, resulting in the advance of nanomedicine. Image-guided drug delivery plays a pivotal role in this field by providing specific targeting as well as image navigation for disease prognosis. Methods: We demonstrate image-guided surgery and drug delivery for the treatment of lung cancer using nanotheranostic H-dots loaded with gefitinib and genistein. Results: The surgical margin for lung tumors is determined by image guidance for precise tumor resection, while targeted anti-cancer drugs function simultaneously for synergistic combination therapy. Compared to conventional chemotherapies, H-dot complexes could improve the therapeutic efficacy of drugs while reducing the risk of adverse effects and drug resistance owing to their ideal biodistribution profiles, high targetability, low nonspecific tissue uptake, and fast renal excretion. Conclusions: These H-dot complexes have unlocked a unique framework for integrating multiple therapeutic and diagnostic modalities into one nanoplatform.
Keywords: Cancer therapy; Combination therapy; Image-guided surgery; Renal clearance; Theranostics.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ. et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–314. - PMC - PubMed
-
- Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322:764–74. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

