The Bufei Jianpi Formula Improves Mucosal Immune Function by Remodeling Gut Microbiota Through the SCFAs/GPR43/NLRP3 Pathway in Chronic Obstructive Pulmonary Disease Rats
- PMID: 35673595
- PMCID: PMC9167601
- DOI: 10.2147/COPD.S359428
The Bufei Jianpi Formula Improves Mucosal Immune Function by Remodeling Gut Microbiota Through the SCFAs/GPR43/NLRP3 Pathway in Chronic Obstructive Pulmonary Disease Rats
Abstract
Purpose: Bufei Jianpi formula (BJF), a traditional Chinese medicine, is an effective and safe therapeutic formula for chronic obstructive pulmonary disease (COPD). BJF treatment is known to reduce the incidence of loose stools in rats with COPD. It is unclear whether BJF regulates gut microbiota. This study examined whether BJF improved mucosal immune function by remodeling the gut microbiota and modulating metabolites in COPD rats.
Methods: Sixty Sprague Dawley (SD) rats were randomized into control, model, BJF, aminophylline (APL), and probiotics (PBT) groups. The stable COPD rat model was duplicated using repeated cigarette smoke inhalation and lipopolysaccharide (LPS) injection. Normal saline, BJF, APL, or PBT were intragastrically administered from weeks eight to twelve, and then the rats were sacrificed at week thirteen. Lung and colon tissues were removed; feces were collected. Pulmonary function, histopathology, levels of inflammatory factors, and activation of NF-κB in the lung tissues were evaluated. Gut microbiota were analyzed using 16S rRNA gene sequencing; fecal short-chain fatty acid (SCFA) concentrations were determined using gas chromatography/mass spectrometry. Mucosal immune response-related genes and proteins were determined using quantitative polymerase chain reaction and Western blotting.
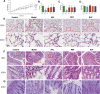
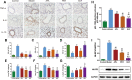
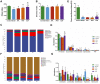
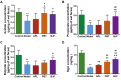
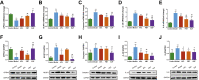
Results: BJF improved pulmonary function and reduced lung inflammation. Further, BJF treatment altered the gut microbiota composition and significantly increased the abundance of Firmicutes and the ratio of Firmicutes to Bacteroides, raising SCFA levels, including acetate, butyrate, and propionate levels. However, the abundance of Bacteroidetes, Proteobacteria, Spirochaetes, Clostridiaceae, and Treponema decreased after BJF administration. BJF decreased the gene and protein expression of NLRP3, Caspase-1, IL-8, and IL-1β, and increased GPR43 expression.
Conclusion: Overall, BJF administration improved mucosal immune function by remodeling the gut microbiota and suppressing the SCFAs/GPR43/NLRP3 pathway in COPD rats. This study provides evidence for the mechanisms underlying BJF-induced improvements in COPD and supports clinical application of BJF.
Keywords: Bufei Jianpi formula; chronic obstructive pulmonary disease; gut microbiota; gut-lung axis; mucosal immune function; short-chain fatty acids.
© 2022 Mao et al.
Conflict of interest statement
The authors report no conflicts of interest in relation to this work and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Health Organization. Mortality and global health estimates. Available from: http://who.int/data/gho/data/themes/mortality-and-global-health-estimates. Accessed November 21, 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

