An efficient CRISPR/Cas9 system for simultaneous editing two target sites in Fortunella hindsii
- PMID: 35673604
- PMCID: PMC9166532
- DOI: 10.1093/hr/uhac064
An efficient CRISPR/Cas9 system for simultaneous editing two target sites in Fortunella hindsii
Abstract
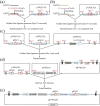
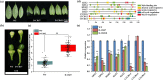
The CRISPR/Cas9 system is a revolutionary genome editing technique and has been widely used in numerous plants. For plants (e.g. citrus) with very low transformation efficiency, how to optimize gene editing efficiency and induce large-fragment deletion has been the focus of research. Here, we report that CRISPR/Cas9 induces efficient deletion of 16-673 bp fragments in the genome of Fortunella hindsii. The ability of two binary vectors, pK7WG2D and pMDC32, to introduce specific mutations into the genome of F. hindsii was evaluated. Double single guide RNAs (sgRNAs) were designed to achieve precise editing of two sites of a gene and deletion of fragments between the two sites. The construction of vectors based on Golden Gate assembly and Gateway recombination cloning is simple and efficient. pK7WG2D is more suitable for F. hindsii genome editing than the pMDC32 vector. Editing efficiency using the pK7WG2D vector reached 66.7%. Allele mutation frequency was 7.14-100%. Plants with 100% allele mutations accounted for 39.4% (13 100% allele mutation plants/33 mutants). The proportion of mutant plants with fragment deletion induced by this editing system was as high as 52.6% (10 fragment-deletion mutants/19 FhNZZ mutants). Altogether, these data suggest that our CRISPR/Cas9 platform is capable of targeted genome editing in citrus and has broad application in research on the citrus functional genome and citrus molecular breeding.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nanjing Agricultural University.
Figures


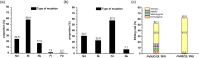
Similar articles
-
Genome sequencing and CRISPR/Cas9 gene editing of an early flowering Mini-Citrus (Fortunella hindsii).Plant Biotechnol J. 2019 Nov;17(11):2199-2210. doi: 10.1111/pbi.13132. Epub 2019 May 21. Plant Biotechnol J. 2019. PMID: 31004551 Free PMC article.
-
Effects of sgRNAs, Promoters, and Explants on the Gene Editing Efficiency of the CRISPR/Cas9 System in Chinese Kale.Int J Mol Sci. 2023 Aug 26;24(17):13241. doi: 10.3390/ijms241713241. Int J Mol Sci. 2023. PMID: 37686051 Free PMC article.
-
Genome Editing in Cotton with the CRISPR/Cas9 System.Front Plant Sci. 2017 Aug 3;8:1364. doi: 10.3389/fpls.2017.01364. eCollection 2017. Front Plant Sci. 2017. PMID: 28824692 Free PMC article.
-
Efficient genome editing of Brassica campestris based on the CRISPR/Cas9 system.Mol Genet Genomics. 2019 Oct;294(5):1251-1261. doi: 10.1007/s00438-019-01564-w. Epub 2019 May 25. Mol Genet Genomics. 2019. PMID: 31129735
-
CRISPR/Cas9; A robust technology for producing genetically engineered plants.Cell Mol Biol (Noisy-le-grand). 2018 Nov 30;64(14):31-38. Cell Mol Biol (Noisy-le-grand). 2018. PMID: 30511631 Review.
Cited by
-
An efficient multiplex approach to CRISPR/Cas9 gene editing in citrus.Plant Methods. 2024 Sep 28;20(1):148. doi: 10.1186/s13007-024-01274-4. Plant Methods. 2024. PMID: 39342225 Free PMC article.
-
CRISPR/Cas9-mediated editing of carotenoid biosynthesis genes alters carotenoid concentrations in kiwifruit.BMC Plant Biol. 2025 Aug 9;25(1):1056. doi: 10.1186/s12870-025-07112-6. BMC Plant Biol. 2025. PMID: 40783509 Free PMC article.
-
Two TAL effectors of Xanthomonas citri promote pustule formation by directly repressing the expression of GRAS transcription factor in citrus.Mol Hortic. 2025 Mar 14;5(1):30. doi: 10.1186/s43897-024-00131-1. Mol Hortic. 2025. PMID: 40083016 Free PMC article.
-
Genome editing for grass improvement and future agriculture.Hortic Res. 2024 Oct 15;12(2):uhae293. doi: 10.1093/hr/uhae293. eCollection 2025 Jan. Hortic Res. 2024. PMID: 39906167 Free PMC article.
-
MdMKK9-Mediated the Regulation of Anthocyanin Synthesis in Red-Fleshed Apple in Response to Different Nitrogen Signals.Int J Mol Sci. 2022 Jul 14;23(14):7755. doi: 10.3390/ijms23147755. Int J Mol Sci. 2022. PMID: 35887103 Free PMC article.
References
-
- Tan B, Li D-L, Xu S-X et al. Highly efficient transformation of the GFP and MAC12.2 genes into precocious trifoliate orange (Poncirus trifoliata [L.] Raf), a potential model genotype for functional genomics studies in citrus. Tree Genet Genomes. 2009;5:529–37.
-
- Zhi J, Liu X, Li D et al. CRISPR/Cas9-mediated SlAN2 mutants reveal various regulatory models of anthocyanin biosynthesis in tomato plant. Plant Cell Rep. 2020;39:799–809. - PubMed
LinkOut - more resources
Full Text Sources

