Clinical outcome and safety of stem cell therapy for ischemic stroke: A systematic review and meta-analysis
- PMID: 35673677
- PMCID: PMC9168316
- DOI: 10.25259/SNI_1174_2021
Clinical outcome and safety of stem cell therapy for ischemic stroke: A systematic review and meta-analysis
Abstract
Background: Several reports on stem cell administration have emerged proving it to be an ideal therapeutic approach for improving neurological functions in ischemic stroke patients. However, some studies also show disappointing results, with some reporting no statistically significant improvements among several different parameters. Several challenges also arise relating to safety and nonscientific aspects, such as ethics.
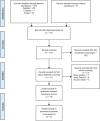
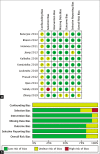
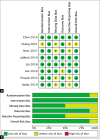
Methods: We performed a systematic review and meta-analysis to evaluate the effect of stem cell therapy on the clinical outcomes of ischemic stroke patients. A systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. A thorough literature search was conducted on PubMed, Scopus, and Cochrane databases. Articles were selected systematically based on the PRISMA protocol and reviewed completely. A total of 19 publications pertaining to stem cell therapy on the ischemic route were included and reviewed. Efficacy outcomes were measured with the National Institutes of Health Stroke Scale, modified Rankin Scale, or Barthel Index.
Results: The results of the meta-analysis indicate that the efficacy outcomes suggest favorable results after stem cell therapy, although not all study results are statistically significant. Stem cell therapy in stroke cases showed a better outcome than standard conservative therapy alone, although our analysis shows that many factors can influence this outcome, and significant effects can only be seen after several months.
Conclusion: The results of this study show promising and satisfying efficacy and a relatively low rate of serious adverse events.
Keywords: Clinical outcome; Ischemic stroke; Safety; Stem cell therapy.
Copyright: © 2022 Surgical Neurology International.
Conflict of interest statement
There are no conflicts of interest.
Figures








Similar articles
-
Comparison of the Administration Route of Stem Cell Therapy for Ischemic Stroke: A Systematic Review and Meta-Analysis of the Clinical Outcomes and Safety.J Clin Med. 2023 Apr 6;12(7):2735. doi: 10.3390/jcm12072735. J Clin Med. 2023. PMID: 37048818 Free PMC article. Review.
-
Meta-Analysis of the Safety and Efficacy of Stem Cell Therapies for Ischemic Stroke in Preclinical and Clinical Studies.Stem Cells Dev. 2019 Apr 15;28(8):497-514. doi: 10.1089/scd.2018.0218. Stem Cells Dev. 2019. PMID: 30739594
-
Efficacy and safety of mesenchymal stem cells in patients with acute ischemic stroke: a meta-analysis.BMC Neurol. 2024 Jan 29;24(1):48. doi: 10.1186/s12883-024-03542-1. BMC Neurol. 2024. PMID: 38287288 Free PMC article.
-
The efficacy and safety of stem cell therapy for ischemic stroke: a systematic review and network meta-analysis study.BMC Neurol. 2025 May 31;25(1):235. doi: 10.1186/s12883-025-04246-w. BMC Neurol. 2025. PMID: 40450234 Free PMC article.
-
Efficacy evaluation of Buyang Huanwu Decoction in the treatment of ischemic stroke in the recovery period: A systematic review of randomized controlled trials.Front Pharmacol. 2022 Oct 14;13:975816. doi: 10.3389/fphar.2022.975816. eCollection 2022. Front Pharmacol. 2022. PMID: 36313307 Free PMC article.
Cited by
-
Efficacy and safety of stem cell therapy for acute and subacute ischemic stroke: a systematic review and meta-analysis.Sci Rep. 2025 Jul 1;15(1):21214. doi: 10.1038/s41598-025-04405-6. Sci Rep. 2025. PMID: 40595869 Free PMC article.
-
X-irradiated umbilical cord blood cells retain their regenerative effect in experimental stroke.Sci Rep. 2024 Mar 22;14(1):6907. doi: 10.1038/s41598-024-57328-z. Sci Rep. 2024. PMID: 38519559 Free PMC article.
-
Mesenchymal stem cell-derived exosomes: Shaping the next era of stroke treatment.Neuroprotection. 2023 Dec;1(2):99-116. doi: 10.1002/nep3.30. Epub 2023 Dec 30. Neuroprotection. 2023. PMID: 38283953 Free PMC article.
-
Comparative study of the efficacy of intra-arterial and intravenous transplantation of human induced pluripotent stem cells-derived neural progenitor cells in experimental stroke.PeerJ. 2023 Nov 9;11:e16358. doi: 10.7717/peerj.16358. eCollection 2023. PeerJ. 2023. PMID: 38025691 Free PMC article.
-
Comparison of the Administration Route of Stem Cell Therapy for Ischemic Stroke: A Systematic Review and Meta-Analysis of the Clinical Outcomes and Safety.J Clin Med. 2023 Apr 6;12(7):2735. doi: 10.3390/jcm12072735. J Clin Med. 2023. PMID: 37048818 Free PMC article. Review.
References
-
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. - PubMed
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics 2019 update: A report from the American heart association. Circulation. 2019;139:e139–596. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
