MLH1, BRAF and p53 - searching for significant markers to predict evolution towards adenocarcinoma in colonic sessile serrated lesions
- PMID: 35673816
- PMCID: PMC9289700
- DOI: 10.47162/RJME.62.4.09
MLH1, BRAF and p53 - searching for significant markers to predict evolution towards adenocarcinoma in colonic sessile serrated lesions
Abstract
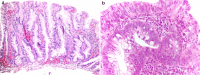
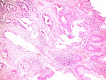
Background and aim: Colonic serrated lesions are premalignant lesions, using an alternative malignization pathway, including multiple genetic and epigenetic alterations, as: mismatch repair deficiency due to MutL homolog 1 (MLH1) promoter methylation, tumor protein p53 (TP53) mutations, activating mutations of v-Raf murine sarcoma viral oncogene homolog B (BRAF) and Kirsten rat sarcoma viral oncogene homolog (KRAS). Our study aims to evaluate MLH1, BRAF and p53 immunohistochemical (IHC) status in sessile serrated lesions (SSLs), with and without dysplasia.
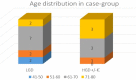
Materials and methods: This is a retrospective case-control study including 20 SSLs with dysplasia and 20 SSLs without dysplasia (matching sex and age). IHC expression of MLH1, BRAF and p53 was evaluated as the percent of nuclear loss of MLH1, cytoplasmic positivity of BRAF and nuclear positivity of p53. Data concerning age, sex, localization of the lesion, dysplasia and IHC results were statistically processed using Microsoft Excel.
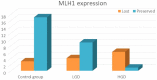
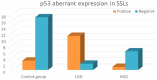
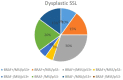
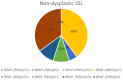
Results: We had very polymorphous patterns of IHC expression for BRAF, MLH1 and p53, especially in the dysplastic group. Thus, two patients were BRAF+∕MLH1-∕p53+, three were BRAF+∕MLH1-∕p53-, one was BRAF+∕MLH1+∕p53- and six were BRAF+∕MLH1+∕p53+. Dysplastic lesions without BRAF mutation exhibited the following phenotype: one case BRAF-∕MLH1-∕p53+, four BRAF-∕MLH1-∕p53- and three BRAF-∕MLH1+∕p53+. In the control group (SSLs without dysplasia), there was a more homogenous distribution of cases: eight cases BRAF+∕MLH1+∕p53-, seven BRAF-∕MLH1+∕p53-, one BRAF-∕MLH1-∕p53+, two BRAF-∕MLH1-∕p53- and two BRAF-∕MLH1+∕p53+.
Conclusions: There are more routes on the serrated pathway, with different mutations and time of acquisition of each genetic or epigenetic lesion with the same morphological result. These lesions should be stratified according to their risk to poor outcome and their need to further surveillance.
Conflict of interest statement
None of the authors have no conflict of interests to disclose.
Figures







Similar articles
-
Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma.Gut. 2017 Jan;66(1):97-106. doi: 10.1136/gutjnl-2015-310456. Epub 2015 Oct 15. Gut. 2017. PMID: 26475632
-
MLH1-deficient Colorectal Carcinoma With Wild-type BRAF and MLH1 Promoter Hypermethylation Harbor KRAS Mutations and Arise From Conventional Adenomas.Am J Surg Pathol. 2016 Oct;40(10):1390-9. doi: 10.1097/PAS.0000000000000695. Am J Surg Pathol. 2016. PMID: 27438990
-
BRAF V600E immunohistochemistry demonstrates that some sessile serrated lesions with adenomatous dysplasia may represent collision lesions.Histopathology. 2019 Jul;75(1):81-87. doi: 10.1111/his.13851. Epub 2019 May 16. Histopathology. 2019. PMID: 30825335
-
Molecular and histologic considerations in the assessment of serrated polyps.Arch Pathol Lab Med. 2015 Jun;139(6):730-41. doi: 10.5858/arpa.2014-0424-RA. Arch Pathol Lab Med. 2015. PMID: 26030242 Review.
-
Pathologic features and biologic importance of colorectal serrated polyps.Adv Anat Pathol. 2009 Mar;16(2):79-91. doi: 10.1097/PAP.0b013e31819923b3. Adv Anat Pathol. 2009. PMID: 19550369 Review.
Cited by
-
The histopathological features and their prognostic impact in the postoperative follow-up of colorectal cancer patients.Rom J Morphol Embryol. 2022 Jul-Sep;63(3):555-561. doi: 10.47162/RJME.63.3.10. Rom J Morphol Embryol. 2022. PMID: 36588494 Free PMC article.
-
Comparative Expression Analysis of TP53 Tumor Suppressor and MDM2 Oncogene in Colorectal Adenocarcinoma.Cancer Diagn Progn. 2024 Mar 3;4(2):129-134. doi: 10.21873/cdp.10298. eCollection 2024 Mar-Apr. Cancer Diagn Progn. 2024. PMID: 38434910 Free PMC article.
-
Clinical and morphopathological assay in vulvovaginal candidiasis.Rom J Morphol Embryol. 2022 Jul-Sep;63(3):511-520. doi: 10.47162/RJME.63.3.05. Rom J Morphol Embryol. 2022. PMID: 36588489 Free PMC article.
References
-
- Bosman FT, Carneiro F, Hruban RH, Theise ND, World Health Organization (WHO) & International Agency for Research on Cancer (IARC), editors. WHO Classification of tumours of the digestive system. 4. Vol. 3. Lyon, France: IARC Press; 2010. pp. 160–165.https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-O...
-
- World Health Organization (WHO) Classification of Tumours Editorial Board, editor. WHO Classification of tumours. Digestive system tumours. 5. Vol. 1. Lyon, France: International Agency for Research on Cancer (IARC) Press; 2019. pp. 163–169.
-
- Niv Y. Changing pathological diagnosis from hyperplastic polyp to sessile serrated adenoma: systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017;29(12):1327–1331. - PubMed
-
- Anderson JC, Lisovsky M, Greene MA, Hagen C, Srivastava A. Factors associated with classification of hyperplastic polyps as sessile serrated adenomas/polyps on morphologic review. J Clin Gastroenterol. 2018;52(6):524–529. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

