B vitamin supply in plants and humans: the importance of vitamer homeostasis
- PMID: 35673947
- PMCID: PMC9544542
- DOI: 10.1111/tpj.15859
B vitamin supply in plants and humans: the importance of vitamer homeostasis
Abstract
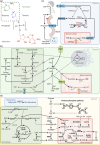
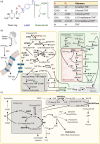
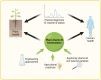
B vitamins are a group of water-soluble micronutrients that are required in all life forms. With the lack of biosynthetic pathways, humans depend on dietary uptake of these compounds, either directly or indirectly, from plant sources. B vitamins are frequently given little consideration beyond their role as enzyme accessory factors and are assumed not to limit metabolism. However, it should be recognized that each individual B vitamin is a family of compounds (vitamers), the regulation of which has dedicated pathways. Moreover, it is becoming increasingly evident that individual family members have physiological relevance and should not be sidelined. Here, we elaborate on the known forms of vitamins B1 , B6 and B9 , their distinct functions and importance to metabolism, in both human and plant health, and highlight the relevance of vitamer homeostasis. Research on B vitamin metabolism over the past several years indicates that not only the total level of vitamins but also the oft-neglected homeostasis of the various vitamers of each B vitamin is essential to human and plant health. We briefly discuss the potential of plant biology studies in supporting human health regarding these B vitamins as essential micronutrients. Based on the findings of the past few years we conclude that research should focus on the significance of vitamer homeostasis - at the organ, tissue and subcellular levels - which could improve the health of not only humans but also plants, benefiting from cross-disciplinary approaches and novel technologies.
Keywords: B vitamins; coenzymes; homeostasis; human health; plant health; vitamer.
© 2022 The Authors. The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest associated with this work.
Figures

Similar articles
-
B Vitamins: An Update on Their Importance for Plant Homeostasis.Annu Rev Plant Biol. 2024 Jul;75(1):67-93. doi: 10.1146/annurev-arplant-060223-025336. Epub 2024 Jul 2. Annu Rev Plant Biol. 2024. PMID: 38424064 Review.
-
Vitamins in plants: occurrence, biosynthesis and antioxidant function.Trends Plant Sci. 2010 Oct;15(10):582-92. doi: 10.1016/j.tplants.2010.07.003. Epub 2010 Aug 21. Trends Plant Sci. 2010. PMID: 20729129 Review.
-
Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients.J Nutr Health Aging. 2006 Sep-Oct;10(5):377-85. J Nutr Health Aging. 2006. PMID: 17066209 Review.
-
Meta-analysis of apparent ruminal synthesis and postruminal flow of B vitamins in dairy cows.J Dairy Sci. 2022 Sep;105(9):7399-7415. doi: 10.3168/jds.2021-21656. Epub 2022 Jul 22. J Dairy Sci. 2022. PMID: 35879170
-
Balancing of B6 Vitamers Is Essential for Plant Development and Metabolism in Arabidopsis.Plant Cell. 2016 Feb;28(2):439-53. doi: 10.1105/tpc.15.01033. Epub 2016 Feb 8. Plant Cell. 2016. PMID: 26858304 Free PMC article.
Cited by
-
The Main Functions of Plastids.Methods Mol Biol. 2024;2776:89-106. doi: 10.1007/978-1-0716-3726-5_5. Methods Mol Biol. 2024. PMID: 38502499 Review.
-
Examination of Primary and Secondary Metabolites Associated with a Plant-Based Diet and Their Impact on Human Health.Foods. 2024 Mar 27;13(7):1020. doi: 10.3390/foods13071020. Foods. 2024. PMID: 38611326 Free PMC article. Review.
-
Variation of vitamin B contents in maize inbred lines: Potential genetic resources for biofortification.Front Nutr. 2022 Oct 21;9:1029119. doi: 10.3389/fnut.2022.1029119. eCollection 2022. Front Nutr. 2022. PMID: 36337650 Free PMC article.
-
Label-free detection of vitamin B by two-step enhanced Raman technique using dynamic borohydride-reduced silver nanoparticles.Mikrochim Acta. 2023 Nov 23;190(12):480. doi: 10.1007/s00604-023-06055-9. Mikrochim Acta. 2023. PMID: 37996711
-
Scientific opinion on the tolerable upper intake level for vitamin B6.EFSA J. 2023 May 17;21(5):e08006. doi: 10.2903/j.efsa.2023.8006. eCollection 2023 May. EFSA J. 2023. PMID: 37207271 Free PMC article.
References
-
- Ajjawi, I. , Milla, M.A.R. , Cushman, J. & Shintani, D.K. (2007) Thiamin pyrophosphokinase is required for thiamin cofactor activation in Arabidopsis. Plant Molecular Biology, 65, 151–162. - PubMed
-
- Akhtar, T.A. , Orsomando, G. , Mehrshahi, P. , Lara‐Nunez, A. , Bennett, M.J. , Gregory, J.F., 3rd et al. (2010) A central role for gamma‐glutamyl hydrolases in plant folate homeostasis. The Plant Journal, 64, 256–266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

