Inactivation of BACE1 increases expression of endothelial nitric oxide synthase in cerebrovascular endothelium
- PMID: 35673977
- PMCID: PMC9536128
- DOI: 10.1177/0271678X221105683
Inactivation of BACE1 increases expression of endothelial nitric oxide synthase in cerebrovascular endothelium
Abstract
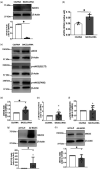
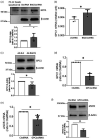
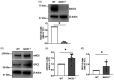
Cerebrovascular effects of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) inactivation have not been systematically studied. In the present study we employed cultured human brain microvascular endothelial cells (BMECs), BACE1-knockout (BACE1-/-) mice and conditional (tamoxifen-induced) endothelium-specific BACE1-knockout (eBACE1-/-) mice to determine effect of BACE1 inhibition on expression and function of endothelial nitric oxide synthase (eNOS). Deletion of BACE1 caused upregulation of eNOS and glypican-1 (GPC1) in human BMECs treated with BACE1-siRNA, and cerebral microvessels of male BACE1-/- mice and male eBACE1-/- mice. In addition, BACE1siRNA treatment increased NO production in human BMECs. These effects appeared to be independent of amyloid β-peptide production. Furthermore, adenoviral-mediated overexpression of BACE1 in human BMECs down-regulated GPC1 and eNOS. Treatment of human BMECs with GPC1siRNA suppressed mRNA and protein levels of eNOS. In basilar arteries of male eBACE1-/- mice, endothelium-dependent relaxations to acetylcholine and endothelium-independent relaxations to NO donor, DEA-NONOate, were not affected, consistent with unchanged expression of eNOS and phosphorylation of eNOS at Ser1177 in large cerebral arteries. In aggregate, our findings suggest that under physiological conditions, inactivation of endothelial BACE1 increases expression of eNOS in cerebral microvessels but not in large brain arteries. This effect appears to be mediated by increased GPC1 expression.
Keywords: Alzheimer’s disease; BACE1; eNOS; endothelial cells; glypican-1.
Conflict of interest statement
Figures



Similar articles
-
BACE2 deficiency impairs expression and function of endothelial nitric oxide synthase in brain endothelial cells.J Neurochem. 2023 Sep;166(6):928-942. doi: 10.1111/jnc.15929. Epub 2023 Aug 7. J Neurochem. 2023. PMID: 37547981 Free PMC article.
-
Cerebrovascular Endothelial Dysfunction: Role of BACE1.Arterioscler Thromb Vasc Biol. 2024 Aug;44(8):1737-1747. doi: 10.1161/ATVBAHA.124.320798. Epub 2024 Jun 13. Arterioscler Thromb Vasc Biol. 2024. PMID: 38868939 Free PMC article. Review.
-
Endothelial BACE1 Impairs Cerebral Small Vessels via Tight Junctions and eNOS.Circ Res. 2022 Apr 29;130(9):1321-1341. doi: 10.1161/CIRCRESAHA.121.320183. Epub 2022 Apr 6. Circ Res. 2022. PMID: 35382554
-
Endothelial nitric oxide modulates expression and processing of amyloid precursor protein.Circ Res. 2010 Dec 10;107(12):1498-502. doi: 10.1161/CIRCRESAHA.110.233080. Epub 2010 Dec 2. Circ Res. 2010. PMID: 21127294 Free PMC article.
-
Endothelial nitric oxide: protector of a healthy mind.Eur Heart J. 2014 Apr;35(14):888-94. doi: 10.1093/eurheartj/eht544. Epub 2013 Dec 18. Eur Heart J. 2014. PMID: 24357508 Free PMC article. Review.
Cited by
-
BACE2 deficiency impairs expression and function of endothelial nitric oxide synthase in brain endothelial cells.J Neurochem. 2023 Sep;166(6):928-942. doi: 10.1111/jnc.15929. Epub 2023 Aug 7. J Neurochem. 2023. PMID: 37547981 Free PMC article.
-
β-secretase 1 overexpression by AAV-mediated gene delivery prevents retina degeneration in a mouse model of age-related macular degeneration.Mol Ther. 2023 Jul 5;31(7):2042-2055. doi: 10.1016/j.ymthe.2023.03.029. Epub 2023 Apr 3. Mol Ther. 2023. PMID: 37016576 Free PMC article.
-
Cerebrovascular Endothelial Dysfunction: Role of BACE1.Arterioscler Thromb Vasc Biol. 2024 Aug;44(8):1737-1747. doi: 10.1161/ATVBAHA.124.320798. Epub 2024 Jun 13. Arterioscler Thromb Vasc Biol. 2024. PMID: 38868939 Free PMC article. Review.
-
Cellular Components of the Blood-Brain Barrier and Their Involvement in Aging-Associated Cognitive Impairment.Aging Dis. 2024 Jul 1;16(3):1513-1534. doi: 10.14336/AD.202.0424. Aging Dis. 2024. PMID: 39122454 Free PMC article. Review.
-
Emerging Roles of Endothelial Nitric Oxide in Preservation of Cognitive Health.Stroke. 2023 Mar;54(3):686-696. doi: 10.1161/STROKEAHA.122.041444. Epub 2023 Feb 27. Stroke. 2023. PMID: 36848426 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

