Cultural adaptation of the Italian version of the Patient-Reported Outcomes Common Terminology Criteria for Adverse Event (PRO-CTCAE®)
- PMID: 35674125
- PMCID: PMC10248298
- DOI: 10.1177/03008916221099558
Cultural adaptation of the Italian version of the Patient-Reported Outcomes Common Terminology Criteria for Adverse Event (PRO-CTCAE®)
Abstract
Introduction: US National Cancer Institute's (NCI) Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE®) is a library of 78 symptom terms and 124 items enabling patient reporting of symptomatic adverse events in cancer trials. This multicenter study used mixed methods to develop an Italian language version of this widely accepted measure, and describe the content validity and reliability in a diverse sample of Italian-speaking patients.
Methods: All PRO-CTCAE items were translated in accordance with international guidelines. Subsequently, the content validity of the PRO-CTCAE-Italian was explored and iteratively refined through cognitive debriefing interviews. Participants (n=96; 52% male; median age 64 years; 26% older adults; 18% lower educational attainment) completed a PRO-CTCAE survey and participated in a semi-structured interview to determine if the translation captured the concepts of the original English language PRO-CTCAE, and to evaluate comprehension, clarity and ease of judgement. Test-retest reliability of the finalized measure was explored in a second sample (n=135).
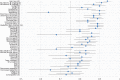
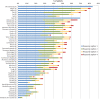
Results: Four rounds of cognitive debriefing interviews were conducted. The majority of PRO-CTCAE symptom terms, attributes and associated response choices were well-understood, and respondents found the items easy to judge. To improve comprehension and clarity, the symptom terms for nausea and pain were rephrased and retested in subsequent interview rounds. Test-retest reliability was excellent for 41/49 items (84%); the median intraclass correlation coefficient was 0.83 (range 0.64-0.94).
Discussion: Results support the semantic, conceptual and pragmatic equivalence of PRO-CTCAE-Italian to the original English version, and provide preliminary descriptive evidence of content validity and reliability.
Keywords: Linguistic validation; PRO-CTCAE; cognitive interviewing; patient-reported outcomes; symptomatic adverse events.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
SN received payments or honoraria from Eli Lilly, Sanofi, Amgen, Beigene, Roche, Takeda, Pfizer, AstraZeneca, Boeringher Ingelheim, MSD, Novartis.
CP received payments or honoraria from Eisai, MSD, BMS, Astra Zeneca, Pfizer, Novartis, Merck, Janssen, Ipsen, EUSA Pharma, Angelini, General Electric.
LDM received payments or honoraria from Roche, Novartis, Eli Lilly, MSD, Pfizer, Ipsen, Celgene, Genomic Helath, Pierre Fabre, Daiichi Sankyo, Seagen, AstraZeneca, Eisai.
GP received payments or honoraria BMS, MSD, Bayer, Astra Zeneca, Merck, Ipsen, Janssen, Novartis, Pfizer, Sanofi.
SC received payments or honoraria from Eli Lilly.
FCal received payments or honoraria from MSD, BMS, Pfizer, AstraZeneca, Ipsen.
GT received payments or honoraria from BMS, MSD, Servier, Astra Zeneca, Merck.
RP received payments or honoraria from Regeneron, Ipsen, Sanofi-Aventis, Amgen, MSD.
FCog received payments or honoraria from Genomic Helath, Biolitec Pharma, Pfizer, Abbott, Celgene, Glaxo, Roche, Bayer, Novartis, Amgen, Pfizer, AstraZeneca, Eisai, Merck-Serono, Boeringher Ingelheim, MSD, BMS, Takeda, Astella, Eli Lilly, Seagen.
VA received payments or honoraria from Amgen, Astra Zeneca, BMS, Eli Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, Sanofi, Servier, Takeda.
DDP received grants to his institution from Fondazione Smith Kline.
EIan received grants to her institution from Fondazione Smith Kline.
FP received payments or honoraria from Incyte, GSK, Eli Lilly, Ipsen, Astellas, Astra Zeneca, Roche, BMS, Bayer, Clovis, Pierre Fabre; FP also received grants to his institution from Roche, Astra Zeneca, Pfizer, MSD, Bayer, Incyte Taiho, Janssen, Exelixis, Aileron, Daiichi Sankyo.
CC, JB, SR, DN, FD, EIez, LS, AFal, VL, AAC, MM, EC, AFer, MBag, MBas, AN, ADA, SAM did not declare competing interests.
Figures

References
-
- National Cancer Institute, National Institutes of Health. US Department of Health and Human Services: Common Terminology Criteria for Adverse Events (CTCAE), https://evs.nci.nih.gov/ftp1/CTCAE/About.html (accessed 25 January 2022).
-
- Fromme EK, Eilers KM, Mori M, et al.. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol 2004; 22: 3485-3490. - PubMed
-
- Basch E, Artz D, Dulko D, et al.. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol 2005; 23: 3552-3561. - PubMed
-
- Kirkova J, Davis MP, Walsh D, et al.. Cancer symptom assessment instruments: a systematic review. J Clin Oncol 2006; 24: 1459-1473. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

