Intra-articular Injection of Mesenchymal Stem Cells After High Tibial Osteotomy in Osteoarthritic Knee: Two-Year Follow-up of Randomized Control Trial
- PMID: 35674255
- PMCID: PMC9216209
- DOI: 10.1093/stcltm/szac023
Intra-articular Injection of Mesenchymal Stem Cells After High Tibial Osteotomy in Osteoarthritic Knee: Two-Year Follow-up of Randomized Control Trial
Abstract
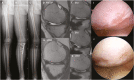
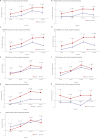
Intra-articular injection of adipose-derived mesenchymal stem cell (ADMSC) after medial open-wedge high tibial osteotomy (MOWHTO) would be a promising disease-modifying treatment by correcting biomechanical and biochemical environment for arthritic knee with varus malalignment. However, there is a paucity of clinical evidence of the treatment. This randomized controlled trial (RCT) was aimed to assess regeneration of cartilage defect, functional improvement, and safety of intra-articular injection of ADMSCs after MOWHTO compared with MOWHTO alone for osteoarthritic knee with varus malalignment. This RCT allocated 26 patients into the MOWHTO with ADMSC-injection group (n = 13) and control (MOWHTO-alone) group (n = 13). The primary outcome was the serial changes of cartilage defect on periodic magnetic resonance imaging (MRI) evaluation using valid measurements until postoperative 24 months. Secondary outcomes were the 2-stage arthroscopic evaluation for macroscopic cartilage status and the postoperative functional improvements of patient-reported outcome measures until the latest follow-up. Furthermore, safety profiles after the treatment were evaluated. Cartilage regeneration on serial MRIs showed significantly better in the ADMSC group than in the control group. The arthroscopic assessment revealed that total cartilage regeneration was significantly better in the ADMSC group. Although it was not significant, functional improvements after the treatment showed a tendency to be greater in the ADMSC group than in the control group from 18 months after the treatment. No treatment-related adverse events, serious adverse events, and postoperative complications occurred in all cases. Concomitant intra-articular injection of ADMSCs with MOWHTO had advantages over MOWHTO alone in terms of cartilage regeneration with safety at 2-year follow-up, suggesting potential disease-modifying treatment for knee OA with varus malalignment.
Trial registration: ClinicalTrials.gov NCT03000712.
Keywords: adipose; derived mesenchymal stem cell; high tibial osteotomy; knee; medial open; osteoarthritis; varus malalignment; wedge high tibial osteotomy.
© The Author(s) 2022. Published by Oxford University Press.
Figures


References
-
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan J.. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 2014;43(6):701-712. 10.1016/j.semarthrit.2013.11.012. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

