Development of an artificial intelligence model for predicting the likelihood of human embryo euploidy based on blastocyst images from multiple imaging systems during IVF
- PMID: 35674312
- PMCID: PMC9340116
- DOI: 10.1093/humrep/deac131
Development of an artificial intelligence model for predicting the likelihood of human embryo euploidy based on blastocyst images from multiple imaging systems during IVF
Abstract
Study question: Can an artificial intelligence (AI) model predict human embryo ploidy status using static images captured by optical light microscopy?
Summary answer: Results demonstrated predictive accuracy for embryo euploidy and showed a significant correlation between AI score and euploidy rate, based on assessment of images of blastocysts at Day 5 after IVF.
What is known already: Euploid embryos displaying the normal human chromosomal complement of 46 chromosomes are preferentially selected for transfer over aneuploid embryos (abnormal complement), as they are associated with improved clinical outcomes. Currently, evaluation of embryo genetic status is most commonly performed by preimplantation genetic testing for aneuploidy (PGT-A), which involves embryo biopsy and genetic testing. The potential for embryo damage during biopsy, and the non-uniform nature of aneuploid cells in mosaic embryos, has prompted investigation of additional, non-invasive, whole embryo methods for evaluation of embryo genetic status.
Study design, size, duration: A total of 15 192 blastocyst-stage embryo images with associated clinical outcomes were provided by 10 different IVF clinics in the USA, India, Spain and Malaysia. The majority of data were retrospective, with two additional prospectively collected blind datasets provided by IVF clinics using the genetics AI model in clinical practice. Of these images, a total of 5050 images of embryos on Day 5 of in vitro culture were used for the development of the AI model. These Day 5 images were provided for 2438 consecutively treated women who had undergone IVF procedures in the USA between 2011 and 2020. The remaining images were used for evaluation of performance in different settings, or otherwise excluded for not matching the inclusion criteria.
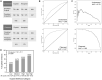
Participants/materials, setting, methods: The genetics AI model was trained using static 2-dimensional optical light microscope images of Day 5 blastocysts with linked genetic metadata obtained from PGT-A. The endpoint was ploidy status (euploid or aneuploid) based on PGT-A results. Predictive accuracy was determined by evaluating sensitivity (correct prediction of euploid), specificity (correct prediction of aneuploid) and overall accuracy. The Matthew correlation coefficient and receiver-operating characteristic curves and precision-recall curves (including AUC values), were also determined. Performance was also evaluated using correlation analyses and simulated cohort studies to evaluate ranking ability for euploid enrichment.
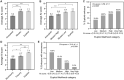
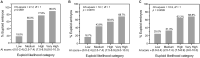
Main results and the role of chance: Overall accuracy for the prediction of euploidy on a blind test dataset was 65.3%, with a sensitivity of 74.6%. When the blind test dataset was cleansed of poor quality and mislabeled images, overall accuracy increased to 77.4%. This performance may be relevant to clinical situations where confounding factors, such as variability in PGT-A testing, have been accounted for. There was a significant positive correlation between AI score and the proportion of euploid embryos, with very high scoring embryos (9.0-10.0) twice as likely to be euploid than the lowest-scoring embryos (0.0-2.4). When using the genetics AI model to rank embryos in a cohort, the probability of the top-ranked embryo being euploid was 82.4%, which was 26.4% more effective than using random ranking, and ∼13-19% more effective than using the Gardner score. The probability increased to 97.0% when considering the likelihood of one of the top two ranked embryos being euploid, and the probability of both top two ranked embryos being euploid was 66.4%. Additional analyses showed that the AI model generalized well to different patient demographics and could also be used for the evaluation of Day 6 embryos and for images taken using multiple time-lapse systems. Results suggested that the AI model could potentially be used to differentiate mosaic embryos based on the level of mosaicism.
Limitations, reasons for caution: While the current investigation was performed using both retrospectively and prospectively collected data, it will be important to continue to evaluate real-world use of the genetics AI model. The endpoint described was euploidy based on the clinical outcome of PGT-A results only, so predictive accuracy for genetic status in utero or at birth was not evaluated. Rebiopsy studies of embryos using a range of PGT-A methods indicated a degree of variability in PGT-A results, which must be considered when interpreting the performance of the AI model.
Wider implications of the findings: These findings collectively support the use of this genetics AI model for the evaluation of embryo ploidy status in a clinical setting. Results can be used to aid in prioritizing and enriching for embryos that are likely to be euploid for multiple clinical purposes, including selection for transfer in the absence of alternative genetic testing methods, selection for cryopreservation for future use or selection for further confirmatory PGT-A testing, as required.
Study funding/competing interest(s): Life Whisperer Diagnostics is a wholly owned subsidiary of the parent company, Presagen Holdings Pty Ltd. Funding for the study was provided by Presagen with grant funding received from the South Australian Government: Research, Commercialisation, and Startup Fund (RCSF). 'In kind' support and embryology expertise to guide algorithm development were provided by Ovation Fertility. 'In kind' support in terms of computational resources provided through the Amazon Web Services (AWS) Activate Program. J.M.M.H., D.P. and M.P. are co-owners of Life Whisperer and Presagen. S.M.D., M.A.D. and T.V.N. are employees or former employees of Life Whisperer. S.M.D, J.M.M.H, M.A.D, T.V.N., D.P. and M.P. are listed as inventors of patents relating to this work, and also have stock options in the parent company Presagen. M.V. sits on the advisory board for the global distributor of the technology described in this study and also received support for attending meetings.
Trial registration number: N/A.
Keywords: ICSI outcome; IVF; PGT-A; artificial intelligence; assisted reproduction; embryo quality; genetics; machine learning; preimplantation genetic testing for aneuploidy.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures
Similar articles
-
Development of an artificial intelligence-based assessment model for prediction of embryo viability using static images captured by optical light microscopy during IVF.Hum Reprod. 2020 Apr 28;35(4):770-784. doi: 10.1093/humrep/deaa013. Hum Reprod. 2020. PMID: 32240301 Free PMC article.
-
Implicit bias in diagnosing mosaicism amongst preimplantation genetic testing providers: results from a multicenter study of 36 395 blastocysts.Hum Reprod. 2024 Jan 5;39(1):258-274. doi: 10.1093/humrep/dead213. Hum Reprod. 2024. PMID: 37873575
-
The use of copy number loads to designate mosaicism in blastocyst stage PGT-A cycles: fewer is better.Hum Reprod. 2023 May 2;38(5):982-991. doi: 10.1093/humrep/dead049. Hum Reprod. 2023. PMID: 36928183
-
Previously reported and here added cases demonstrate euploid pregnancies followed by PGT-A as "mosaic" as well as "aneuploid" designated embryos.Reprod Biol Endocrinol. 2023 Mar 8;21(1):25. doi: 10.1186/s12958-023-01077-7. Reprod Biol Endocrinol. 2023. PMID: 36890559 Free PMC article. Review.
-
Preimplantation genetic testing for aneuploidies (abnormal number of chromosomes) in in vitro fertilisation.Cochrane Database Syst Rev. 2020 Sep 8;9(9):CD005291. doi: 10.1002/14651858.CD005291.pub3. Cochrane Database Syst Rev. 2020. PMID: 32898291 Free PMC article.
Cited by
-
Clinical Prospects for Artificial Intelligence in Obstetrics and Gynecology.JMA J. 2025 Jan 15;8(1):113-120. doi: 10.31662/jmaj.2024-0197. Epub 2024 Dec 13. JMA J. 2025. PMID: 39926075 Free PMC article. Review.
-
Application of a methodological framework for the development and multicenter validation of reliable artificial intelligence in embryo evaluation.Reprod Biol Endocrinol. 2025 Jan 31;23(1):16. doi: 10.1186/s12958-025-01351-w. Reprod Biol Endocrinol. 2025. PMID: 39891250 Free PMC article.
-
Non-invasively predicting euploidy in human blastocysts via quantitative 3D morphology measurement: a retrospective cohort study.Reprod Biol Endocrinol. 2024 Oct 28;22(1):132. doi: 10.1186/s12958-024-01302-x. Reprod Biol Endocrinol. 2024. PMID: 39468586 Free PMC article.
-
The impact of low oocyte maturity ratio on blastocyst euploidy rate: a matched retrospective cohort study.Contracept Reprod Med. 2024 Aug 26;9(1):41. doi: 10.1186/s40834-024-00303-w. Contracept Reprod Med. 2024. PMID: 39187878 Free PMC article.
-
The use of voting ensembles to improve the accuracy of deep neural networks as a non-invasive method to predict embryo ploidy status.J Assist Reprod Genet. 2023 Feb;40(2):301-308. doi: 10.1007/s10815-022-02707-6. Epub 2023 Jan 14. J Assist Reprod Genet. 2023. PMID: 36640251 Free PMC article.
References
-
- Capalbo A, Rienzi L.. Mosaicism between trophectoderm and inner cell mass. Fertil Steril 2017;107:1098–1106. - PubMed
-
- Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Nagy ZP, Ubaldi FM.. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod 2014;29:1173–1181. - PubMed
-
- Chavez-Badiola A, Flores-Saiffe-Farías A, Mendizabal-Ruiz G, Drakeley AJ, Cohen J.. Embryo Ranking Intelligent Classification Algorithm (ERICA): artificial intelligence clinical assistant predicting embryo ploidy and implantation. Reprod Biomed Online 2020;41:585–593. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

